��Ŀ����
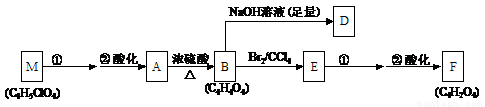
����ѧһһѡ��ѧ�뼼�������Ṥ���ŷŵ�β���У��������Ķ�������Ϊ��ֹ��Ⱦ���������ŷ�ǰ�������β���������跨�����ۺ����ã�
��1�����Ṥ���ŷ�β���е�SO2ͨ��������ʯ��ˮ���գ�Ȼ������ϡ���ᴦ����
��д���������̵Ļ�ѧ��Ӧ����ʽ��
����˵������β�������������ŵ㣨˵�����㼴�ɣ�
����ij���᳧ÿ���ŷŵ�1����3������״����β���к�0.2%������ٷ�������SO2��ͨ��������������������������ʯ��
��2�������������ձ������о���Na2SO3���շ���Ϊ����SO2��Ⱦ��һ���·������÷�������һ������Na2SO3ˮ��Һ����SO2���ڶ����Ǽ���������Һ���ɵõ�����Ũ��SO2��ˮ��������Ʒ��
����β�����������루1����ȵ��ŵ���
��3��ij�о�С����NaOH��Һ����β���еĶ����������õ�Na2SO3����Һ���е��ѭ�������������¹��ս�����ѭ��������������Ĥ���ѭ������������Aͼ��a��b���ӽ���Ĥ�����۷�Ϊ�������缫����Ϊʯī��
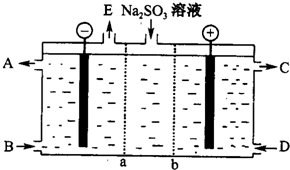
��ͼ��a��ʾ
�������ĵ缫��ӦʽΪ
��������1��������SO2���ʼ��ɽ��
�ڿɴ�ԭ�ϼ����ã�SO2�������ýǶȷ�����
�����ù�ϵʽ����ɵã�
��2��д�������ķ�Ӧ����ʽ���۲�ɵã�
��3���ٴ�CΪ�����֪��bΪ�����ӽ���Ĥ����aΪ�����ӽ���Ĥ����������ӦΪˮ�ŵ���������������������AΪ�������ƣ�EΪ������
������ӦΪ��������ŵ������������д�����ɣ�
�ڿɴ�ԭ�ϼ����ã�SO2�������ýǶȷ�����
�����ù�ϵʽ����ɵã�
��2��д�������ķ�Ӧ����ʽ���۲�ɵã�
��3���ٴ�CΪ�����֪��bΪ�����ӽ���Ĥ����aΪ�����ӽ���Ĥ����������ӦΪˮ�ŵ���������������������AΪ�������ƣ�EΪ������
������ӦΪ��������ŵ������������д�����ɣ�
����⣺��1����SO2������ʯ��ˮ��Ӧ������������ƺ�ˮ��������ƺ����ᷴӦ��������ơ�ˮ�Ͷ����������Ļ�ѧ��Ӧ�ֱ�ΪSO2+Ca��OH��2=CaSO3��+H2O��CaSO3+H2SO4=CaSO4+SO2��+H2O��
�ʴ�Ϊ��SO2+Ca��OH��2=CaSO3��+H2O��CaSO3��+H2SO4=CaSO4+SO2��+H2O��
��ԭ����ʯ�ҡ�����۸����������ã��ɵõ�ʯ�ั��Ʒ��������SO2�����ϸ߿ɷ�����Ϊԭ�ϣ�
�ɴ�Ϊ��ԭ����ʯ�ҡ�����۸����������ã��ɵõ�ʯ�ั��Ʒ�������SO2�����ϸ߿ɷ�����Ϊԭ�ϣ�
��SO2 ��CaO
64 56
1��107L��0.2%��22.4L/mol��64g/mol m�� CaO��
64��56=1��107L��0.2%��22.4L/mol��64g/mol��m�� CaO��
���m�� CaO��=50000g=50kg��
�ʴ�Ϊ��50��
��2����Na2SO3+H2O+SO2=2NaHSO3��2NaHSO3=Na2SO3+H2O+SO2�����۲�������Ӧ��֪Na2SO3��ѭ��ʹ�ã�
�ʴ�Ϊ��Na2SO3��ѭ��ʹ�ã�
��3���ٴ�CΪ�����֪���������Դ����������ŵ磮��bΪ�����ӽ���Ĥ��aΪ�����ӽ���Ĥ����������ӦΪˮ�ŵ��������������������ӣ���AΪ�������ƣ�EΪ������
�ʴ�Ϊ������NaOH��Һ��������
������ӦΪ��������ŵ��������������Ӧ�����ӷ���ʽΪSO32--2e-+H2O=2H++SO42-��
�ʴ�Ϊ��SO32--2e-+H2O=2H++SO42-��
�ʴ�Ϊ��SO2+Ca��OH��2=CaSO3��+H2O��CaSO3��+H2SO4=CaSO4+SO2��+H2O��
��ԭ����ʯ�ҡ�����۸����������ã��ɵõ�ʯ�ั��Ʒ��������SO2�����ϸ߿ɷ�����Ϊԭ�ϣ�
�ɴ�Ϊ��ԭ����ʯ�ҡ�����۸����������ã��ɵõ�ʯ�ั��Ʒ�������SO2�����ϸ߿ɷ�����Ϊԭ�ϣ�
��SO2 ��CaO
64 56
1��107L��0.2%��22.4L/mol��64g/mol m�� CaO��
64��56=1��107L��0.2%��22.4L/mol��64g/mol��m�� CaO��
���m�� CaO��=50000g=50kg��
�ʴ�Ϊ��50��
��2����Na2SO3+H2O+SO2=2NaHSO3��2NaHSO3=Na2SO3+H2O+SO2�����۲�������Ӧ��֪Na2SO3��ѭ��ʹ�ã�
�ʴ�Ϊ��Na2SO3��ѭ��ʹ�ã�
��3���ٴ�CΪ�����֪���������Դ����������ŵ磮��bΪ�����ӽ���Ĥ��aΪ�����ӽ���Ĥ����������ӦΪˮ�ŵ��������������������ӣ���AΪ�������ƣ�EΪ������
�ʴ�Ϊ������NaOH��Һ��������
������ӦΪ��������ŵ��������������Ӧ�����ӷ���ʽΪSO32--2e-+H2O=2H++SO42-��
�ʴ�Ϊ��SO32--2e-+H2O=2H++SO42-��
���������⿼���˶����������Ⱦ�����Ρ����ԭ������ѧ����ʽ����д����ϵʽ�ļ��㣮��Ŀ��3����һ���Ѷȣ������������

��ϰ��ϵ�д�
�����Ŀ
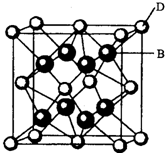 ����ѧһһѡ�����ʽṹ�����ʡ�
����ѧһһѡ�����ʽṹ�����ʡ�
 ��A��C��Ԫ�ص����� ����д��ѧʽ��
��A��C��Ԫ�ص����� ����д��ѧʽ��
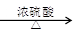 (i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6
(i) ��25��ʱ������ƽ�ⳣ��K1��3.9��10��4��K2��5.5��10��6 (ii)
A + RCOOH(��ROH)
(ii)
A + RCOOH(��ROH) ����ζ�IJ���
����ζ�IJ���