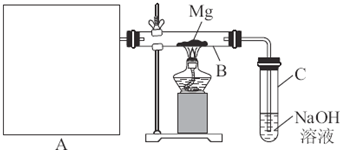
��Ŀ����
��֪SiO2��SO2��CO2���������������ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ�������ԡ��������»�ѧʵ�鷽������Ƽ�Ҫ�����ʡ��Ʊ��ͼ���ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣

��1��ѡ����ȡSO2�ĺ����Լ�____________________________________________��
��10����H2SO4��Һ ��98����H2SO4��Һ ��Na2SO3���� ��CaSO3���� ��Cu
��2��д��װ��B�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ___________________________________��
װ��C��NaOH��Һ��������_______________________________________________��
��3������Ϊ��װ���Ƿ��в���֮����________________________________������У���һһ˵��____________________________________________________________��
��ij�о�С������ˡ�ʵ������Si�����о��������Կα�Ϊ�������������ϵõ����¿ɹ��ο�����Ϣ��
�ٹ�ҵ���ڸ���ʱ��C��ԭSiO2���Ƶ�Si ��Mg�ڵ�ȼ�������¼�����SiO2��Ӧ �۽����軯����ϡH2SO4��Ӧ������������SiH4 ��Si��SiO2������ϡH2SO4��Ӧ ��SiH4�ڿ�������ȼ
�������о������м����š�����ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡ�����ܽ������Ȼ����ˡ�ϴ�ӡ������������������ϡ�����ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��63�����ҡ���
��4����С�顰ʵ������Si���Ļ�ѧ����ʽ��_________________________________________��
��5������ơ���ϡ�����ܽ�������ʱ�������б������ͻ���ԭ����_________________
____________________________________________________________________��
(1) �ڢ�
(2)SO2+Mg![]() 2MgO+S ���ն����SO2����ֹSO2��Ⱦ����
2MgO+S ���ն����SO2����ֹSO2��Ⱦ����
(3)�� ��A��B֮��ȱ�ٸ���װ�ú�Cװ���������ͨ
(4)2Mg+SiO2![]() 2MgO+Si
2MgO+Si
(5)����þ�����ɵĹ������Ӧ�õ��軯þ���軯þ�����ᷴӦ����SiH4����ȼ
���û�ѧ����ʽ˵����
2Mg + Si====Mg2Si
Mg2Si + 2H2SO4====2MgSO4 + SiH4��
SiH4 + 2O2====SiO2 + 2H2O
��������.��1��������ȡSO2��A�����ȣ�����ȡSO2���Լ�ֻ����98%ŨH2SO4��Na2SO3���塣��2��SO2��Mg��Ӧ�Ļ�ѧ����ʽΪSO2+2Mg![]() 2MgO+S��C��NaOH��������β���е�SO2����ֹ����Ⱦ��������3�����װ�õIJ���֮���У�A��B��ȱ�ٸ���װ�ã�Cװ���������ͨ��
2MgO+S��C��NaOH��������β���е�SO2����ֹ����Ⱦ��������3�����װ�õIJ���֮���У�A��B��ȱ�ٸ���װ�ã�Cװ���������ͨ��
��.��4��SiO2+2Mg![]() 2MgO+Si����5����������Si��Mg��Ӧ���ɵ�Mg2Si����H2SO4�ܽ�����з�����Ӧ��Mg2Si+2H2SO4====2MgSO4+SiH4����SiH4����ȼ�����³��ֱ������ͻ�
2MgO+Si����5����������Si��Mg��Ӧ���ɵ�Mg2Si����H2SO4�ܽ�����з�����Ӧ��Mg2Si+2H2SO4====2MgSO4+SiH4����SiH4����ȼ�����³��ֱ������ͻ�