��Ŀ����
(8��)(1)��(N2H4)�Ƿ��亽��ɴ����õĸ���ȼ�ϡ���NH3��NaClO��һ�����ʵ����Ȼ�Ϸ�Ӧ�������¡�NaCl��ˮ���÷�Ӧ�Ļ�ѧ����ʽ��_____________________________��
(2)�ڻ���ƽ�����װ��ǿ��ԭ����(N2H4)��ǿ�������������⣬�����ǻ��ʱ���������������壬���ų������ȡ���֪��H2O(l) H2O(g) ��H= +44 kJ/mol��12.8 gҺ̬���������������ⷴӦ���ɵ�����ˮ�������ų�256.65 kJ��������
����д��Һ̬����������ⷴӦ����Һ̬ˮ���Ȼ�ѧ����ʽ______________________��
����16 g Һ̬���������������ⷴӦ����Һ̬ˮʱ�ų���������___________________��
(1)2NH3+NaClO=N2H4+NaCl+H2O
(2)��2H4(l)+2H2O2(l)=N2(g)+4H2O(l) H=-817.625kJ/mol
��08.8125kJ
��������
�������������⿼������й��Ȼ�ѧ��Ӧ�е�һЩ֪ʶ���ڷ�Ӧ���漰��������ԭ��Ӧ���й�֪ʶ���ڷ�Ӧ�е�ʧ������ȣ����ԣ�(1)2NH3+NaClO=N2H4+NaCl+H2O���ڵڶ��������У�Ҫ��д������Һ̬�е�ʱ����Ȼ�ѧ����ʽ��12.8g���µ����ʵ���Ϊ0.4mol��������N2H4(l)+2H2O2(l)=N2(g)+4H2O(l) H=-817.625kJ/mol
���㣺�Ȼ�ѧ���й�֪ʶ��

 ��У����ϵ�д�
��У����ϵ�д�(6��)ͨ���Ѳ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ���. ��֪���ֻ�ѧ���ļ������£�
| ��ѧ�� | N��H | N��N | O==O | N��N | O��H |
| ����(kJ/mol) | 386 | 167 | 498 | 946 | 460 |
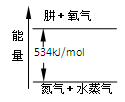
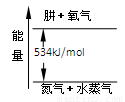
(2)Ϊ�������(N2H4)ȼ�չ������ͷŵ����������ö������������������������������߷�Ӧ���ɵ�����ˮ�������ң�
��N2(g)��2O2(g)===2NO2(g)����H1����67.7 kJ/mol
��N2H4(g)��O2(g)===N2(g)��2H2O(g)����H2����534 kJ/mol
��д���º�NO2��ȫ��Ӧ���Ȼ�ѧ����ʽ��____________________________________
(3)�����й��ռ似���ķ�չ���ж���2009��Я��̽���ǹ��̣�Ѱ�Ҹ���Ч�Ļ���ƽ���Ҳ���ᵽ�������ճ̣���ʵ�����ҹ�������ԱӦ�õ��Ӽ����ģ������и�����������N60�����Ľṹ��C60ʮ�����ƣ���֪N60������ÿ��Nԭ�Ӿ��Ե��������������ԭ�ӣ���N60���ӽṹ��ÿ����ԭ�Ӿ��γ�8�����ӵ��ȶ��ṹ�����Ʋ�1��N60�Ľṹ����________��N��N����