��Ŀ����
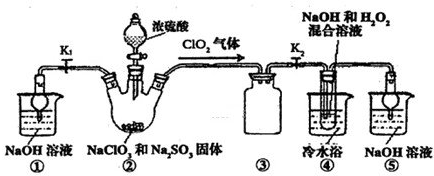
ij��ѧ��ȤС��ͬѧ�������и�����װ����ȡ��ϩ�����ù�����������C2H4������CO2��H2O���ⶨ��ϩ��̼��������Ԫ�ص������ȣ�
�ش��������⣺
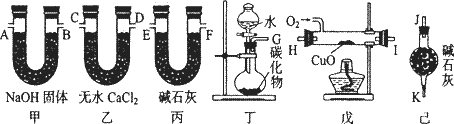
(1)��ͬѧ��ʵ�����Ʊ�C2H2�����������CaC2��ZnC2��Al4C3��Mg2C2��Li2C2��ѡ��һ���Լ���ˮ��Ӧ���Ʊ���ϩ��д���÷�Ӧ�Ļ�ѧ����ʽ��_________��
(2)������������������������װ�õ��ܵ�����˳���ǣ�G��E��F��_________��_________��_________��_________��A��B��J��
(3)װ�ü�������_________��
װ�ü���������_________��
(4)װ������CuO��������_________��
(5)ʵ��ǰ�Ƶüס�����װ�õ������ֱ�Ϊm1 g��n1 g��ʵ����ϣ��Ƶüס�����װ�õ������ֱ��Ϊm2 g��n2 g������ϩ������̼ԭ�Ӻ���ԭ�ӵ�ԭ�Ӹ�����Ϊ_________(�г���ʽ)��
(6)������û��CuO����ⶨ���_________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)�������ӱ�װ�ã���ⶨ���_________��
�𰸣�

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
ij��ѧ��ȤС��ͬѧ�ܡ���-����-��ˮ��ء�������������ʦ��ָ�����������ޣ����Ͻ𣩡���ͥ���õ�Ư��ˮ��ʳ�Ρ��������ơ�ʯī�缫���ӷϾɸɵ���л�ã���ԭ��������һ��ԭ��أ���ص��ܷ�Ӧ����ʽΪ2A1+3C10-?+20H-??3C1-?+2Al
+H20������˵������ȷ���ǣ�������
| O | - 2 |
A���õ�صĸ�����ӦʽΪ2Al+80H-?-6e-??2Al
| ||
| B���õ缫��������ӦʽΪ3C10-?+3H20+6e-?3Cl-?+60H-? | ||
| C�����·�е��Ӵ�ʯī�缫���������� | ||
| D������0.1 mol Al��ȫ�ܽ�ʱ���������·�ĵ��Ӹ���ԼΪ1.806��1023 |
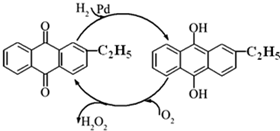 ������������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȣ�ij��ѧ��ȤС��ͬѧΧ���Ź������չ�˵����о���ʵ�飮
������������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȣ�ij��ѧ��ȤС��ͬѧΧ���Ź������չ�˵����о���ʵ�飮