��Ŀ����
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ�� ��֪����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH�����й��л���ķе����±���
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
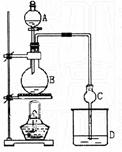 A�зŴ��ᣬB�н��������Ҵ�������Ũ�����ϣ�D�з��б���̼������Һ������Һ©���ߵμӴ��ᡢ���ȡ�
A�зŴ��ᣬB�н��������Ҵ�������Ũ�����ϣ�D�з��б���̼������Һ������Һ©���ߵμӴ��ᡢ���ȡ�
��ش�
��1����Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����_________________��
��2���÷�Ӧ�У�����CH3CH218OH�����ᷢ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
��3��������C��������______________________________��
����Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ���ǣ������ӷ���ʽ��ʾ��_______________________����Ӧ������D�е�������____________________________��
��4����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ������_______________���ټ��루�˿մ�����ѡ����ѡ��______ ___��
Ŀ���� ��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A������������ B����ʯ��
C����ˮ������ D����ʯ��
��1������һ�ַ�Ӧ�������������Ӧ�������
��2��CH3COOH + CH3CH218OH ![]() CH3CO18OCH2CH3 + H2O
CH3CO18OCH2CH3 + H2O
��3�������������� CO32-+H2O ![]() HCO3-+OH��
HCO3-+OH��
��Һ�ֲ㣬�ϲ�Ϊ��ɫ��״Һ�壬�²���Һ��ɫ�ɺ�ɫ��Ϊ��ɫ�����dz��
��4���Ҵ� C����ˮ�����ƣ� ��ˮ ��ÿ��2�֣�

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д�



