��Ŀ����
��16�֣����ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ��Ҫ������ԭ����Ư����������ο����������������ӵĹ��׳�������
��1��40 ��ʱ���һ�������0.1 mol/L H2C2O4��Һ��һ�����0.01 mol/L����KMnO4��Һ����д���пո�
| �¶� | v(H2C2O4) | v(KMnO4) | KMnO4��ɫʱ�� |
| 40 �� | 10 ml | 10 ml | 40 s |
| 40 �� | 20 ml | 20 ml |
|
��2���ü�����ָʾ����ͨ�����ζ��ɲⶨ������ҺŨ�ȡ�������һ�ֳ��õ����ָʾ�������ȣ��û�ѧʽHIn��ʾ����������һ���л����ᣬ����뷽��ʽΪ��HInH++In�������������In������ɫ�� �����ȵ������������ȣ� ����ǰ��ǿ��ǰ������ʮ�ֽӽ������жϣ���
��3����������pH��Ϊ3������Ͳ�����Һ��10 ml����ˮϡ�ͣ��ڴ�����ϻ���ϡ������������Һ��c(H+)�仯��ʾ��ͼ������Ҫ�ı�ע��
��4��������MgC2O4��Ksp=8.1��10-5����֪����Һ��ij���ӵ�Ũ�ȡ�10-5 mol/Lʱ����Ϊ�������ѳ�����ȫ����Ϊ�˳���1 L0.01 mol/LMgCl2��Һ�е�Mg2+������100 mL0.1 mol/L��(NH4)2C2O4��Һ��ͨ�������ж�Mg2+�Ƿ��ѳ�����ȫ��
��5��(NH4)2C2O4��Һ��NH4+ˮ��̶��Դ���C2O42-���������ԣ�0.1 mol/L(NH4)2C2O4��ҺpHֵ6.4������ijδ֪��Һ����������ˮ�������c(H+)=1.0��10-5 mol/L������ҺpH������ ������ĸ����
A��5 B��6 C��7 D��9
��1��40 s ��2�֣�
��2����ɫ ǰ��������2�֣�
��3����ͼ��3�֣���㡢�յ��1�֣�б��1�֣�
��4��MgCl2��(NH4)2C2O4ǡ����ȫ��Ӧ������
MgC2O4(s)Mg2+(aq)+C2O42-(aq)��c(Mg2+)��c(C2O42-)=Ksp=8.1��10-5��
��c(Mg2+)=c(C2O42-)��
c(Mg2+)=(8.1��10-5)-1/2=9��10-3 mol/L>10-5mol/L
��Mg2+δ������ȫ����4�֣�
��5��ABCD ��3�֣�ѡ��1����1�֣�ѡ��2����2�֣�ѡ��3�����ϵ�3�֣�
����:��1����Ӧ�����뷴Ӧ���Ũ���йأ��뷴Ӧ������ʵ��������أ����������ص�Ũ�Ȳ�û�з����仯�������¶�Ҳû�иı䣬���Է�Ӧ�����Dz���ģ�����ɫʱ���Dz���ġ�
��2�����ȵı�ɫ��Χ��3.1��4.4��С��3.1ʱ�Ժ�ɫ������4.4ʱ�Ի�ɫ�����ݼ��ȵĵ��뷽��ʽ��֪������Һ������ǿʱƽ����������Ӧ�����ƶ��ģ����Ը��������ɫ���Ի�ɫ�ġ���Ϊ��������Һˮ���Լ��ԣ���ʱ�����Ի�ɫ����������������ڼ��ȵģ����ڲ�����Һ�м����Ի�ɫ������ͨ����ɫ�ı仯���жϵζ��յ㡣��˲��������Ҫǿ�ڼ��ȵģ������ڵζ���������ɫ���ܷ����仯��
��3��������ǿ�ᣬ���������ᣬ���ڵ���ƽ�⣬������ϡ�����������������ӵ�Ũ��ʼ��С�ڲ����������ӵ�Ũ�ȡ���������ϡ��ʱ���ߵ�PH��Ҫ���ӽ�7��
��4��1 L0.01 mol/LMgCl2��Һ�е�Mg2+�����ʵ�����0.01mol��100 mL0.1 mol/L��(NH4)2C2O4��Һ�����ʵ���Ҳ��0.01mol��������ǡ�÷�Ӧ�������ܽ�ƽ��MgC2O4(s)Mg2+(aq)+C2O42-(aq)��֪c(Mg2+)��c(C2O42-)=Ksp=8.1��10-5
��Ϊc(Mg2+)=c(C2O42-)������c(Mg2+)=(8.1��10-5)-1/2=9��10-3 mol/L>10-5 mol/L
��Mg2+δ������ȫ��
��5����������ˮ�������c(H+)=1.0��10-5 mol/L��˵������Һ��ˮ�ĵ����DZ��ٽ��ģ���ֻ��������ˮ�⣬����Һ�����ԣ�����ʱ��Һ��pHΪ5����ֻ��������ˮ�⣬����Һ�Լ��ԣ���ʱ��Һ�е�c(OH��)��1.0��10��5 mol/L������Һ�е�c(H+)=1.0��10��9 mol/L������Һ��pH��9������Һ�����Ӻ�������ͬʱ��ˮ�⡣�������ӵ�ˮ��̶ȴ��������ӵ�ˮ��̶ȣ�����Һ�Լ��ԣ��������ӵ�ˮ��̶�С�������ӵ�ˮ��̶ȣ�����Һ�����ԣ������ӵ�ˮ��̶Ⱥ������ӵ�ˮ��̶���ͬ������Һ�����ģ�����ѡ��A��B��C��D����ȷ��




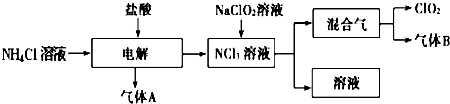
 �ö������ȣ�ClO2�����������ƣ�Na2FeO4Ħ������Ϊ166g?mol-1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl-��Fe3+��
�ö������ȣ�ClO2�����������ƣ�Na2FeO4Ħ������Ϊ166g?mol-1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl-��Fe3+��