��Ŀ����
ҽ��ͨ����������ע��Һά�ֲ���ѪҺ�е�Ѫ�Ǻ�������ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��
| ������ע��Һ |
| ���250mL |
| �ܶȣ�1.08g?mL-1 |
| �������ţ�10032032 |
| ��Ч�ڣ���2013��3�� |
| ����������5% |
��1��ʵ�黹ȱ�ٵ�������______��
��2�����ж�����ƿ����ʹ�÷�������������ȷ����______��
A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
B������ƿ������ˮϴ�������ñ�������ע��Һ��ϴ
C��������Һʱ�����ƺõ������Ǿ���С�ĵ�������ƿ�У�������ˮ����̶���1��2cmʱ�����ý�ͷ�ιܼ�����ˮ���̶���
D������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
��3��ʵ������ȡ��______ g���壬���ƺ����Һ�����ʵ����ʵ���Ũ��Ϊ______��
��4������һ�����ʵ���Ũ�ȵ���������Һʱ�����в���ʹʵ����ƫ�͵���______��
A������ƿ��ԭ����������ˮ����������B��ϴ���ձ��Ͳ���������Һδת������ƿ�У�
C������ʱ�۲�Һ�温�ӡ�������������D������ʱ�۲�Һ������
E�����ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ��������Һ��Ũ�ȣ�
�⣺��1��������һ�����ʵ���Ũ��NaOH��Һ�����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡҩƷ������С�ձ��г�����Ȼ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�500ml����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ���ձ���ҩ�ס�������������ƿ����ͷ�ιܣ��ʴ�Ϊ��500ml����ƿ��
��2��A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ����A��ȷ��
B������ƿ������������Һ�ģ�����Ҫ��������ע��Һ��ϴ����B����
C��������ƿ����ֱ�������ܽ���壬��C����
D������ƿ�ϱ����ݻ����¶Ⱥ��D����
��ѡ��A��
��3�������ǣ�C6H12O6��ע��Һ���ܶ�Ϊ1.08g?mL-1��500mL��Һ�������ǵ���������5%���������ǵ�����Ϊ��500mL��1.08g/cm3��5%=27.0g�������ǵ����ʵ���Ϊ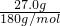 =0.15mol�����ʵ����ʵ���Ũ��Ϊ
=0.15mol�����ʵ����ʵ���Ũ��Ϊ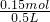 =0.3mol/L���ʴ�Ϊ��27.0��0.3mol/L��
=0.3mol/L���ʴ�Ϊ��27.0��0.3mol/L��
��4��A������ƿ��ԭ����������ˮ������������ʵ�������Ӱ�죬��������ҺŨ����Ӱ�죬��A����
B��ϴ���ձ��Ͳ���������Һδת������ƿ�У���������ƿ�����ʵ����ʵ�����С��������Һ��Ũ��ƫ�ͣ���B��ȷ��
C������ʱ�۲�Һ�温�ӣ�����������Һ�����ƫС��������Һ��Ũ��ƫ��C����
D������ʱ�۲�Һ�����ӣ�����������Һ�����ƫ��������Һ��Ũ��ƫС����D��ȷ��
E�����ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ�����������Һ�����ƫ��������Һ��Ũ��ƫС����E��ȷ��
��ѡ��BDE��
��������1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2��A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ��
B������ƿ������������Һ�ģ�����Ҫ��������ע��Һ��ϴ��
C����������ƿ����ֱ�������ܽ���壻
D������ƿ�ϱ����ݻ����¶Ⱥ��
��3������������ܶȿɼ�����Һ�������������������ǵ��������������������ǵ�����������c=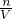 �����㣻
�����㣻
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=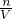 ����������������ҺŨ�ȵ�Ӱ�죻
����������������ҺŨ�ȵ�Ӱ�죻
���������⿼��һ�����ʵ���Ũ����Һ�����Ƽ����������Ѷ��еȣ�ע�����c=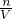 ������Һ��������������
������Һ��������������
��2��A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ����A��ȷ��
B������ƿ������������Һ�ģ�����Ҫ��������ע��Һ��ϴ����B����
C��������ƿ����ֱ�������ܽ���壬��C����
D������ƿ�ϱ����ݻ����¶Ⱥ��D����
��ѡ��A��
��3�������ǣ�C6H12O6��ע��Һ���ܶ�Ϊ1.08g?mL-1��500mL��Һ�������ǵ���������5%���������ǵ�����Ϊ��500mL��1.08g/cm3��5%=27.0g�������ǵ����ʵ���Ϊ
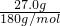 =0.15mol�����ʵ����ʵ���Ũ��Ϊ
=0.15mol�����ʵ����ʵ���Ũ��Ϊ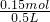 =0.3mol/L���ʴ�Ϊ��27.0��0.3mol/L��
=0.3mol/L���ʴ�Ϊ��27.0��0.3mol/L�� ��4��A������ƿ��ԭ����������ˮ������������ʵ�������Ӱ�죬��������ҺŨ����Ӱ�죬��A����
B��ϴ���ձ��Ͳ���������Һδת������ƿ�У���������ƿ�����ʵ����ʵ�����С��������Һ��Ũ��ƫ�ͣ���B��ȷ��
C������ʱ�۲�Һ�温�ӣ�����������Һ�����ƫС��������Һ��Ũ��ƫ��C����
D������ʱ�۲�Һ�����ӣ�����������Һ�����ƫ��������Һ��Ũ��ƫС����D��ȷ��
E�����ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ�����������Һ�����ƫ��������Һ��Ũ��ƫС����E��ȷ��
��ѡ��BDE��
��������1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2��A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ��
B������ƿ������������Һ�ģ�����Ҫ��������ע��Һ��ϴ��
C����������ƿ����ֱ�������ܽ���壻
D������ƿ�ϱ����ݻ����¶Ⱥ��
��3������������ܶȿɼ�����Һ�������������������ǵ��������������������ǵ�����������c=
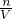 �����㣻
�����㣻��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=
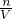 ����������������ҺŨ�ȵ�Ӱ�죻
����������������ҺŨ�ȵ�Ӱ�죻���������⿼��һ�����ʵ���Ũ����Һ�����Ƽ����������Ѷ��еȣ�ע�����c=
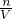 ������Һ��������������
������Һ�������������� 
��ϰ��ϵ�д�
�����Ŀ
 2FeCl3��������Ԫ�صĻ��ϼ�________������ߡ����͡�������________�����������ԭ������ͬ����Cl2��________��������________�ԣ��ڸ÷�Ӧ�У���������1mol Fe��������________mol FeCl3��
2FeCl3��������Ԫ�صĻ��ϼ�________������ߡ����͡�������________�����������ԭ������ͬ����Cl2��________��������________�ԣ��ڸ÷�Ӧ�У���������1mol Fe��������________mol FeCl3�� Al2O3+2Fe������Fe2O3��
Al2O3+2Fe������Fe2O3��