��Ŀ����
����Ŀ�������о���ѧϰС��ֱ����̽���Ե�ʵ�飺
���飺Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ���������ͼ��ʾ��ʵ��װ�á�
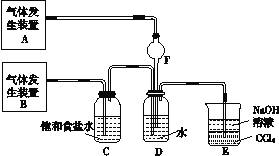
��1�����巢��װ��A�в��������廯ѧʽΪ_______________________________��
��2��װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��3��װ��E��������___________________________________________________��
���飺Ϊ�˷ֱ��о�SO2��Cl2�����ʣ��������ͼ��ʾ��ʵ��װ�á�
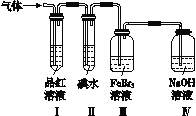
��4����ͨ��SO2ʱ��װ�����з�����Ӧ�����ӷ���ʽΪ________________���÷�Ӧ��SO2���ֳ�__________�ԡ�
��5����װ������װ��5.0 mL 1.0��10��3 mol��L��1��ˮ����ͨ������Cl2��ȫ��Ӧ��ת����5.0��10��5 mol���ӣ���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��6������Ϊ������Ƶ�װ���Ƿ��в���֮����__________(����������������)������У���д���Ľ�������______________________________(����ޣ��˿ղ���)��
���𰸡� SO2 Cl2��SO2��2H2O=2HCl��H2SO4 ��ֹ���������ն����SO2��Cl2 2Fe3����SO2��2H2O=2Fe2����SO42����4H�� ��ԭ I2��5Cl2��6H2O=10HCl��2HIO3 �� װ�â�ȥ���������ƿ�����ӻ���˫��������һ�ײ���һ��ֱ����
����������1����װ��ͼ��֪��Bװ���������ɺ�ͨ�뱥��ʳ��ˮ�У�˵����Cl2�ķ���װ�ã�ͨ��ʳ��ˮ��ȥ�����е�HCl���壬����Aװ�ò���������ΪSO2��SO2��������ˮ�����壬ͨ��Fͨ��ˮ��ֹ��������2�������Ͷ���������������ԭ��Ӧ�����Ȼ�������ᣬ��Ӧ����ʽΪ��Cl2+SO2+2H2O�T2HCl+H2SO4����3�������Ͷ��������ж�������ֱ���ſգ������߶��ܺ�����������Һ��Ӧ��Eװ���к��л���װ�ã��ܷ�ֹ����������β��������������������Һ���������Ͷ�������ֹ��������4��III�������Ӿ��������ԣ��ܺͶ���������������Ӧ����������������ӣ����ӷ�Ӧ����ʽΪ��2Fe3����SO2��2H2O=2Fe2����SO42����4H�����÷�Ӧ�У���Ԫ��ʧ���ӻ��ϼ����ߣ����Զ�����������ԭ�������ֳ���ԭ�ԣ���5��װ��B��װ��5.0mL 1.0mol?L-1�ĵ�ˮ����ͨ������Cl2��ȫ��Ӧ��ת����5.0��10-2mol���ӣ���IԪ�������������еĻ��ϼ�Ϊ+![]() =+5������������ΪHIO3����������ԭΪHCl����Ӧ����ʽΪ��I2��5Cl2��6H2O=10HCl��2HIO3����6��β������װ���У��������ѹǿ���������ӳ��������ȫ�������䴦������Ϊ��װ�â�ȥ���������ƿ�����ӻ���˫��������һ�ײ���һ��ֱ������
=+5������������ΪHIO3����������ԭΪHCl����Ӧ����ʽΪ��I2��5Cl2��6H2O=10HCl��2HIO3����6��β������װ���У��������ѹǿ���������ӳ��������ȫ�������䴦������Ϊ��װ�â�ȥ���������ƿ�����ӻ���˫��������һ�ײ���һ��ֱ������

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ��ֿɳ�Ϊȼ�ϡ���ҵ�����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H
CH3OH(g) ��H
��CO2(g)+3H2(g)![]() CH3OH��g��+H2O(g) ��H����58 kJ/mol
CH3OH��g��+H2O(g) ��H����58 kJ/mol
��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H����41 kJ/mol
CO(g)+H2O(g) ��H����41 kJ/mol
�ش��������⣺
��1����֪��Ӧ���е���صĻ�ѧ�������������£�
��ѧ�� | H��H | C��O | C | H��O | C��H |
E/��kJ.mol-1�� | 436 | 343 | 1076 | 465 | x |
��x��_________��
��2����T��ʱ��6molCO2��8molH2����2L�ܱ������з�����Ӧ�ڣ����H2�����ʵ�����ʱ��仯��ͼ��״̬��(ͼ��ʵ��)��ʾ��ͼ������A(1��6)������1minʱH2�����ʵ�����6mol��

��T��ʱ״̬�������£�0��3min��CH3OH��ƽ����Ӧ����v��_____________mol/(L��min)��ƽ�ⳣ��K��____��
��������������ʱ�����ı�ijһ��������H2�����ʵ�����ʱ��仯��ͼ��״̬����ʾ����ı������������_______��
�������������䣬���ı��¶�ʱ�����H2�����ʵ�����ʱ��仯��ͼ��״̬����ʾ����״̬����Ӧ���¶�________(������������������=��)T�棻
����״̬����ƽ�ⳣ��ΪK2��״̬����ƽ�ⳣ��ΪK3����K2____(����������������������)K3��
��һ���¶��£��˷�Ӧ�ں��������н��У����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬���ݵ���_____��
a��������ѹǿ���� b���״���ˮ����������ȱ��ֲ���
c��v����H2����3v�棨CH3OH�� d��2��C��O���ѵ�ͬʱ��6��H��H����