��Ŀ����
��ͼΪһЩ���ʼ���ת���ϵ����Ӧ���ڹ�ҵ�Ͽ���������������C����Ӧ���ڹ�ҵ�Ͽ�����������J��Na2FeO4������Ӧ�١��ڡ��ܺ͢ݾ�����ˮ��Һ�н��еķ�Ӧ�������£�D��E��G�������壬B����ɫҺ�壻F��ˮ��Һ����Ϊɱ����������H��һ������ʯ����Ҫ�ɷ֣���������Ԫ����ɣ���������Ԫ�ص���������Ϊ70%��
��ش��������⣺
��1����ҵ�����÷�Ӧ������������C���õ�������C�ĵ缫������______��
��2��������F�������ӵĵ���ʽΪ______��
��3����Ӧ�ڵĻ�ѧ����ʽΪ______��
��4����Ӧ�ݵ����ӷ���ʽΪ______��
��5���������ƣ�Na2FeO4������ɱ����������һ�֡���ɫ������Ч���ľ�ˮ������ԭ��Ϊ����______����______��
������J��Na2FeO4����
��1�������������������������ж����������������ڵĵ缫��
��2��F�Ǵ������ƣ�д������������ӵĵ���ʽ��
��3���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��4���������Ӻʹ�����������ڼ��������·�Ӧ���ɸ������Ρ������Ӻ�ˮ��
��5�����ݸ������Ƶ����ʷ������
����⣺��1������Ȼ�����Һʱ�������������������������������������������������д������������ӣ��ʵõ��������������Ƶĵ缫������������
�ʴ�Ϊ��������
��2��F�Ǵ������ƣ��������Ƶ��������Ǵ���������ӣ�����ʽΪ��
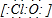 ��
���ʴ�Ϊ��
 ��
����3���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ��Cl2+2NaOH=NaCl+NaClO+H2O��
��4���������Ӻʹ�����������ڼ��������·�Ӧ���ɸ������Ρ������Ӻ�ˮ��
��Ӧ�����ӷ���ʽΪ��2Fe3++3ClO-+10OH-=2FeO42-+3Cl-+5H2O��
�ʴ�Ϊ��2Fe3++3ClO-+10OH-=2FeO42-+3Cl-+5H2O��
��5���������ƣ�Na2FeO4������ǿ�����ԣ�������ɱ���������������Ʊ���ԭΪ���������ӣ�����������ˮ������������������������ˮ�е�������������γɳ�����ʹˮ���壮
�ʴ�Ϊ���������ƣ�Na2FeO4������ǿ�����ԣ��������Ʊ���ԭΪ���������ӣ�����������ˮ������������������������ˮ�е�������������γɳ�����ʹˮ���壮
���������⿼����Ԫ�ػ�������ƶϡ����ԭ����֪ʶ�㣬�ѶȲ�����ݴ������ǿ�����Ժͽ�������ʷ����������Ƶ����ʣ�

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�(һ)(1)ijѧ����������ƽ����һ��С�ձ�����������С�ձ�������Ϊ32.6g���á�������ʾ�������Ϸ����룬��������ʾ��������ȡ���룬���ü�ͷ���±�����գ���ʾ�������̣�����ͼ3��2��ʾ��������ϻ��������λ��(����|����ʾ)��
| ��������/g | 50 | 20 | 20 | 10 | 5 |
| ȡ��������� |

(2)ͼ3��3��ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ4����Ͳ�е�Һ��������__________mL��
(3)����![]() ��������Һ200mL����IJ�������Ϊ___________��
��������Һ200mL����IJ�������Ϊ___________��
(��)���ſ�ѧ�����ķ�չ�������ӵ������IJⶨ�ֶ�Խ��Խ�࣬�ⶨ�ľ�ȷ��ҲԽ��Խ�ߡ�����һ�ּ��еIJⶨ�ķ��������岽��Ϊ��
(1)������NaClϸ�������ȷ��ȡmg NaCl���岢ת�Ƶ���������A�У�
(2)�õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶ȣ������NaCl��������![]() ��
��
��ش��������⣺
�ٲ���(1)��A�������ʹ��___________��(�����)
A����Ͳ B���ձ� C������ƿ D���Թ�
�ڲ���(2)������ʽ�ζ��ܻ����ü�ʽ�ζ���______________��������_____________��
���ܷ���ˮ���汽__________��������____________��
����֪NaCl�����У����������![]() ��
��![]() ���ƽ������Ϊa cm(��ͼ3��4)���������ⶨ������õİ����ӵ�����
���ƽ������Ϊa cm(��ͼ3��4)���������ⶨ������õİ����ӵ�����![]() �ı���ʽΪ_______________��
�ı���ʽΪ_______________��

(��)���������ʵ���Ũ����Һʱ�����в������ֵĺ����(�����ҺŨ�Ȳ�ȷ������ƫ�͡�����ƫ�ߡ�������Ӱ�족)��
(1)��������������Һʱ����ȡ���������������ƹ��塣_____________��
(2)��������������Һʱ����Һ���ܳ��壬�����������ܽ⡣____________��
(3)�����Ȼ�����Һʱ������ƿ��������ˮ��______________��
(4)����ʱ��Һ���Ϸ���̶�����ʱ��ֹͣ��ˮ��__________��
(5)���ƺ���Һ������ƿδ���ã�����һЩ��Һ��_____________��
(6)������ҺҺ�泬���̶��ߣ���������������ˮ��ʹҺ�潵���̶���________________��