��Ŀ����
(6��)�ҹ�����Ϊ��������ȱ�������涨��ʳ���б�����������ĵ���ء�����ʳ�����Ƿ�ӵ⣬���������·�Ӧ��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O
(1)�á�˫���š���ʾ��������Ӧ�е���ת�Ƶķ������Ŀ
________________________________________��
(2)�����Ӧ��ת��0��2mol���ӣ�������I2�����ʵ���Ϊ_________________��
(3)����������Ӧ����ʳ�����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)
![]() �ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
�ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
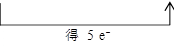 ��1��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O
��1��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O
��2��0.12mol ��3���ڢۢ�
����:��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 SO4====3K2SO4+3I2+3H2O
SO4====3K2SO4+3I2+3H2O �����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)
�����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)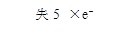 �ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
�ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ