��Ŀ����
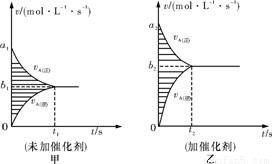
��2mol A��1mol B����ij�ܱ������з�����Ӧ��2A��g��+B��g��?xC��g�����ﵽ��ѧƽ���C���������Ϊa������÷�Ӧ�������ֱ�����и�ѡ���������ͬ�������ж���ȷ���ǣ�������
���������ݵ�Чƽ���֪ʶ����⣬�ڵ�Чƽ���бȽϳ���������Ҫ��������Ҫ���������֣���һ�����º����£����ڷ�Ӧǰ��������������仯�ķ�Ӧ��˵���ȼ�ת����Ӧ��������ʼͶ�ϵ����ʵ�����ԭƽ����ʼ̬��ͬ����ƽ���Ч�����������º����£����ڷ�Ӧǰ���������û�б仯�ķ�Ӧ��˵���ȼ�ת����ֻҪ��Ӧ�������������ʵ����ı�����ԭƽ����ʼ̬��ͬ����ƽ���Ч�������� ���º�ѹ�£�����������ϵ��Чת����ֻҪ��Ӧ�������������ʵ����ı�����ԭƽ����ʼ̬��ͬ����ƽ���Ч��
����⣺A���ں��º�ѹ�£��ı���ʼʱ�������ʵ����ʵ�����ֻҪ����ѧ������֮�Ȼ���ɻ�ѧ����ʽ��ͬһ�����ʵ����ʵ���֮����ԭƽ����ͬ���ﵽƽ��״̬����ԭƽ���Ч����x=1ʱ����1.5 mol A��1 mol C��Ϊ��ʼ�ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ����ֱ�Ϊ2.5mol��1mol������Ӧ��2molA��1molB�Ļ���ﲻ�ǵ�Ч�ģ�ƽ���C���������һ��������a����A����
B�����º����£���x=2ʱ����2molC��Ϊ��ʼ�ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ��������൱����ʼʱ��2 molA��1molB������ʼ����2molA��1molB��ȣ�Ϊ��Чƽ�⣬ƽ���C�����������Ϊa����B��ȷ��
C���ں��º�ѹ�£��ı���ʼʱ�������ʵ����ʵ�����ֻҪ����ѧ������֮�Ȼ���ɻ�ѧ����ʽ��ͬһ�����ʵ����ʵ���֮����ԭƽ����ͬ���ﵽƽ��״̬����ԭƽ���Ч����x=3ʱ1 mol A��1 mol B��6 mol C����ʼ���ʣ�ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ��������൱����ʼʱ��5molA��3molB������ʼ����2molA��1molB��A��B�ı�Ϊ2��1���ﵽƽ��״̬����ԭƽ�ⲻ��Ч������C���������������a����C����
D�����ں��º����£���0.6 mol A��0.3 mol B��1.4 mol C����ʼ���ʣ�����=2��ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ����ֱ�Ϊ2molA��1molB����ԭ��Ӧ��Ч��������x=3ʱ����Ӧ����ʽ���ߵ����廯ѧ������֮����ȣ�ѹǿ��Ӱ��ƽ�⣬���ڼ����A��B�����ʵ�������2��1���ﵽƽ��ʱ��ԭƽ��Ҳ�ǵ�Чƽ�⣬����x=2��x=3�����ԣ���D����
��ѡB��
B�����º����£���x=2ʱ����2molC��Ϊ��ʼ�ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ��������൱����ʼʱ��2 molA��1molB������ʼ����2molA��1molB��ȣ�Ϊ��Чƽ�⣬ƽ���C�����������Ϊa����B��ȷ��
C���ں��º�ѹ�£��ı���ʼʱ�������ʵ����ʵ�����ֻҪ����ѧ������֮�Ȼ���ɻ�ѧ����ʽ��ͬһ�����ʵ����ʵ���֮����ԭƽ����ͬ���ﵽƽ��״̬����ԭƽ���Ч����x=3ʱ1 mol A��1 mol B��6 mol C����ʼ���ʣ�ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ��������൱����ʼʱ��5molA��3molB������ʼ����2molA��1molB��A��B�ı�Ϊ2��1���ﵽƽ��״̬����ԭƽ�ⲻ��Ч������C���������������a����C����
D�����ں��º����£���0.6 mol A��0.3 mol B��1.4 mol C����ʼ���ʣ�����=2��ͨ����Ӧ�Ļ�ѧ������֮�Ȼ����A��B�����ʵ����ֱ�Ϊ2molA��1molB����ԭ��Ӧ��Ч��������x=3ʱ����Ӧ����ʽ���ߵ����廯ѧ������֮����ȣ�ѹǿ��Ӱ��ƽ�⣬���ڼ����A��B�����ʵ�������2��1���ﵽƽ��ʱ��ԭƽ��Ҳ�ǵ�Чƽ�⣬����x=2��x=3�����ԣ���D����
��ѡB��
���������⿼���˵�Чƽ���֪ʶ������Ĺؼ�������ʱע�������������������Ӧ�ķ�����⣮�����á��ȼ�ת�����ķ����������ͽ����Чƽ�����⣬�����ѶȽϴ�

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ

 3C(g)��D(s)����֪��2mol A��1mol
B����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��C�����ʵ���ΪWmol��C��ƽ���������е��������Ϊn%��
3C(g)��D(s)����֪��2mol A��1mol
B����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��C�����ʵ���ΪWmol��C��ƽ���������е��������Ϊn%��