��Ŀ����
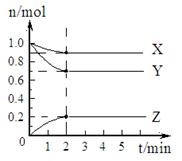
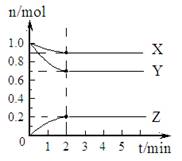
 ��1��ij�¶��£���һ�ܱ������У�X��Y��Z ������������ʵ�����ʱ��ı仯��������ͼ��ʾ�������й����ݣ�д��X��Y��Z��Ӧ�Ļ�ѧ����ʽ
��1��ij�¶��£���һ�ܱ������У�X��Y��Z ������������ʵ�����ʱ��ı仯��������ͼ��ʾ�������й����ݣ�д��X��Y��Z��Ӧ�Ļ�ѧ����ʽX+3Y?2Z
X+3Y?2Z
����2�������������֤����1���еķ�Ӧ�Ѵﵽƽ��״̬����
B��C��D
B��C��D
������ţ���A�����ʵ���Ũ�ȣ�c��X��=c��Y��=c��Z��
B���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯
C���¶Ⱥ����һ��ʱ�������ڵ�ѹǿ���ٱ仯
D���¶Ⱥ����һ��ʱ����������ƽ����Է����������ٱ仯
��3��ij�¶��£�����һ���Ϊ2L���ܱ������г���2mol X��3mol Y��Ȼ��1���еĻ�ѧ����ʽ���з�Ӧ�����ﵽ��ѧƽ��ʱ�����Z�����ʵ����İٷֺ���Ϊ25%����ƽ��ʱX�����ʵ���Ũ��Ϊ
0.75mol/L
0.75mol/L
����������1���������ʵ����ı仯�жϷ�Ӧ���������������ʵ����ı仯֮�ȵ��ڻ�ѧ������֮����д����ʽ��
��2����ѧ��Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�ɴ�������һЩ���������䣬�Դ˷�����
��3�����ݷ�Ӧ����ʽ��������ʽ�����㣮
��2����ѧ��Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�ɴ�������һЩ���������䣬�Դ˷�����
��3�����ݷ�Ӧ����ʽ��������ʽ�����㣮
����⣺��1����ͼ����Կ�������Ӧ��X��Y�����ʵ�����С��Z�����ʵ������࣬��X��YΪ��Ӧ�ZΪ������ҡ�n��Y������n��X������n��Z��=0.3mol��0.1mol��0.2mol=3��1��2����Ӧ�Ļ�ѧ����ʽΪ��X+3Y 2Z��
2Z��
�ʴ�Ϊ��X+3Y 2Z��
2Z��
��2��A����Ӧƽ��ʱ��Ũ��ȡ���ڷ�Ӧ��ʼʱ��Ũ�Ⱥ�ת���ij̶ȣ����������ж��Ƿ�ƽ��״̬����A����
B���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯��˵���ﵽƽ��״̬����B��ȷ��
C�����ڷ�Ӧǰ������Ļ�ѧ��������ͬ�����¶Ⱥ����һ��ʱ�������ڵ�ѹǿ���ٱ仯������˵���ﵽƽ��״̬����C��ȷ��
D�����ڷ�Ӧǰ������Ļ�ѧ��������ͬ���¶Ⱥ����һ��ʱ����������ƽ����Է����������ٱ仯������˵���ﵽƽ��״̬����D��ȷ��
�ʴ�Ϊ��B��C��D��
��3����ƽ��ʱ��X��Ũ�ȵı仯Ϊx��
X+3Y 2Z
2Z
��ʼ��1mol/L 1.5mol/L 0
ת����x 3x 2x
ƽ�⣺��1mol/L-x����1.5mol/L-3x��2x
��
��100%=25%��
��֮�ã�x=0.25mol/L��
���ԣ�ƽ��ʱX�����ʵ���Ũ��Ϊ1mol/L-0.25mol/L=0.75mol/L��
�ʴ�Ϊ��0.75mol/L
 2Z��
2Z���ʴ�Ϊ��X+3Y
 2Z��
2Z����2��A����Ӧƽ��ʱ��Ũ��ȡ���ڷ�Ӧ��ʼʱ��Ũ�Ⱥ�ת���ij̶ȣ����������ж��Ƿ�ƽ��״̬����A����
B���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯��˵���ﵽƽ��״̬����B��ȷ��
C�����ڷ�Ӧǰ������Ļ�ѧ��������ͬ�����¶Ⱥ����һ��ʱ�������ڵ�ѹǿ���ٱ仯������˵���ﵽƽ��״̬����C��ȷ��
D�����ڷ�Ӧǰ������Ļ�ѧ��������ͬ���¶Ⱥ����һ��ʱ����������ƽ����Է����������ٱ仯������˵���ﵽƽ��״̬����D��ȷ��
�ʴ�Ϊ��B��C��D��
��3����ƽ��ʱ��X��Ũ�ȵı仯Ϊx��
X+3Y
 2Z
2Z��ʼ��1mol/L 1.5mol/L 0
ת����x 3x 2x
ƽ�⣺��1mol/L-x����1.5mol/L-3x��2x
��
| 2x |
| 1-x+1.5-3x+2x |
��֮�ã�x=0.25mol/L��
���ԣ�ƽ��ʱX�����ʵ���Ũ��Ϊ1mol/L-0.25mol/L=0.75mol/L��
�ʴ�Ϊ��0.75mol/L
���������⿼���Ϊ�ۺϣ��漰��ѧ����ʽ���жϣ�ƽ��״̬���ж��Լ�ƽ��Ũ�ȵļ��㣬��Ŀ�ѶȲ���ע����ؼ��㷽����Ӧ�ã�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ