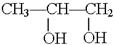��Ŀ����
�����廯����A������ͼ��ת����ϵ��
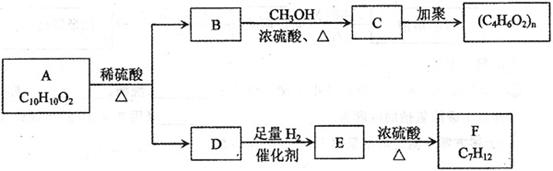
��֪D����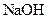 ��Һ��Ӧ��Eת��ΪFʱ������ֻ��һ�ֽṹ������ʹ������Ȼ�̼��Һ��ɫ����ش��������⣺
��Һ��Ӧ��Eת��ΪFʱ������ֻ��һ�ֽṹ������ʹ������Ȼ�̼��Һ��ɫ����ش��������⣺
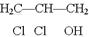
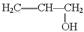
��1��B�ķ���ʽ��_____________
��2��A��F�Ľṹ��ʽ A ___________ F __________
��3��д��B��C��E��F�Ļ�ѧ��Ӧ����ʽ����ע����Ӧ����
B��C��_____________________________________________�� ��
E��F��_____________________________________________�� ��
��4����������3��������A��ͬ���칹���ж���
�ٱ������жԶ�ȡ���ṹ ���� ��Һ����ɫ
��Һ����ɫ
���ܷ���������Ӧ
��д����������һ�ֵĽṹ��ʽ________________________________________

��֪D����
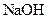 ��Һ��Ӧ��Eת��ΪFʱ������ֻ��һ�ֽṹ������ʹ������Ȼ�̼��Һ��ɫ����ش��������⣺
��Һ��Ӧ��Eת��ΪFʱ������ֻ��һ�ֽṹ������ʹ������Ȼ�̼��Һ��ɫ����ش��������⣺��1��B�ķ���ʽ��_____________
��2��A��F�Ľṹ��ʽ A ___________ F __________
��3��д��B��C��E��F�Ļ�ѧ��Ӧ����ʽ����ע����Ӧ����
B��C��_____________________________________________�� ��
E��F��_____________________________________________�� ��
��4����������3��������A��ͬ���칹���ж���
�ٱ������жԶ�ȡ���ṹ ����
 ��Һ����ɫ
��Һ����ɫ ���ܷ���������Ӧ
��д����������һ�ֵĽṹ��ʽ________________________________________
��1��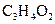 �� ��2�֣�
�� ��2�֣�
��2��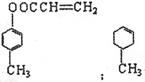 ����2�֣���4�֣�
����2�֣���4�֣�
��3��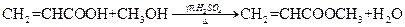 ��3�֣���ȡ����1�֣�
��3�֣���ȡ����1�֣�
��4��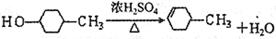 ��3�֣�����ȥ��1�֣�
��3�֣�����ȥ��1�֣�
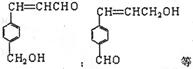 ������������Ҳ���֣�2�֣�
������������Ҳ���֣�2�֣�
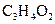 �� ��2�֣�
�� ��2�֣���2��
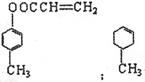 ����2�֣���4�֣�
����2�֣���4�֣���3��
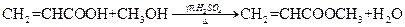 ��3�֣���ȡ����1�֣�
��3�֣���ȡ����1�֣���4��
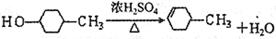 ��3�֣�����ȥ��1�֣�
��3�֣�����ȥ��1�֣� ������������Ҳ���֣�2�֣�
������������Ҳ���֣�2�֣���

��ϰ��ϵ�д�
�����Ŀ
 �����Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������ƣ���Ӧ������������________������ţ�1�֣���
�����Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������ƣ���Ӧ������������________������ţ�1�֣��� ��CH3CH��CH CH3
��CH3CH��CH CH3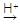 CH3CHBrCH3 + CH3CH2CH2Br
CH3CHBrCH3 + CH3CH2CH2Br (��Ҫ����)
(��Ҫ����)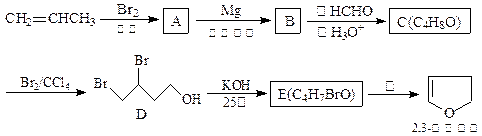


 ��
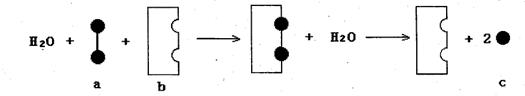
��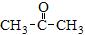 �Լ�D����������ͼ��ʾ��
�Լ�D����������ͼ��ʾ��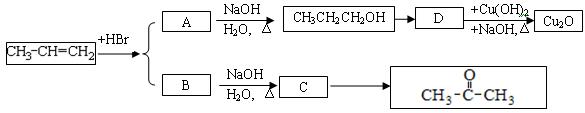
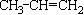 ��Ӧ����A�Ļ�ѧ����ʽΪ___________________________________��
��Ӧ����A�Ļ�ѧ����ʽΪ___________________________________��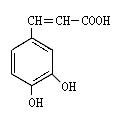


 ��
��
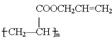 ����
����