��Ŀ����
����Ŀ��H2�ڹ�ũҵ����������������Ҫ�����á�
I����1����ҵ���õ�ⱥ��ʳ��ˮ�ķ�����ȡH2���÷�Ӧ�����ӷ���ʽΪ___________��
��2����ͼ��ΪLiBH4/MgH2��ϵ�����ʱ�ʾ��ͼ��

����LiH��B��H2��Ӧ�õ�LiBH4���Ȼ�ѧ����ʽΪ_________��
�����������ƣ�Na2FeO4����һ�����Ͷ�ܲ��ϣ���ҵ��Na2FeO4�Ʊ��������Դ�������ʯī�缫Ϊ�����缫�����и�Ĥ�ĵ��ۣ���ͼ��ʾ�������ŨNaOH��Һ�Ʊ��������ƣ���ѧ����ʽΪ��Fe+2NaOH+2H2O= Na2FeO4+2H2��

��3��AΪ��Դ_______��������������������)�����缫_____��������������������������ʯī�缫��
��4��ʯī�缫�Ϸ����ĵ缫��ӦʽΪ______��
��5���������п��ܴ��ڸ���������ʱ�����ɺ��ɫ��������ʱֻҪ�Ӵ����ǿ�ȣ��Ϳ���ʹ���ɫ����������⣬ת��ΪFeO42-���ù��̵ĵ缫��ӦʽΪ____
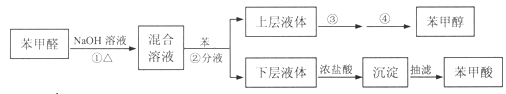
������6�����ü״�ȼ�ϵ�ؽ��е���װ����ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ���Ͽ�K����ʱA��B���������ɵ����ʵ��������塣

����B�缫Ϊ_____���������������������������õ缫�����ɵ������ڱ�״���µ����Ϊ__________��
���𰸡�2Cl-+2H2O![]() 2OH-+Cl2��+H2�� 2LiH(s)+2B(s)+3H2(g)====2LiBH4(s)��H=-200kJ��mol-1 �� ���� H++2e-=H2����2H2O+2e-=H2��+2OH- Fe(OH)3-3e-+5OH-==FeO42-+4H2O ���� 2. 24 L
2OH-+Cl2��+H2�� 2LiH(s)+2B(s)+3H2(g)====2LiBH4(s)��H=-200kJ��mol-1 �� ���� H++2e-=H2����2H2O+2e-=H2��+2OH- Fe(OH)3-3e-+5OH-==FeO42-+4H2O ���� 2. 24 L
��������
(1)��ҵ�õ�ⱥ��ʳ��ˮ�ķ�����ȡNaOH��ͬʱ����H2��Cl2��
(2)��ͼ��֪��2LiBH4(s)+MgH2(s)=2LiH(s)+2B(s)+MgH2(s)+3H2(g)��H=+200 kJmol-1���ݴ˷������
(3)��ʯī�������缫���ŨNaOH��Һ�Ʊ���������(Na2FeO4)��ԭ������ʧ�������ɻ�ԭ�Ե����ӣ�Ȼ���������������ɸ������ƣ�
(4)�����У��������Ϸ����õ��ӵĻ�ԭ��Ӧ��
(5)������Ϣ�����ɫ����������⣬ת��ΪFeO42-���ݴ���д�缫��Ӧ��
(6)��Ϊԭ��أ�ͨ��״��ĵ缫Ϊ������ͨ�������ĵ缫Ϊ��������ACΪ������BDΪ������B�缫Ϊ����������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ����B�ĵ缫��ӦΪ��Cu2++2e-=Cu��2H++2e-=H2����A�缫Ϊ�������缫��ӦΪ��4OH--4e-=2H2O+O2�������ݵ�ʧ�����غ���ʽ���㡣
I��(1)��ҵ�õ�ⱥ��ʳ��ˮ�ķ�����ȡNaOH��ͬʱ����H2��Cl2����ѧ����ʽΪ��2Cl-+2H2O![]() 2OH-+Cl2��+H2�����ʴ�Ϊ��2Cl-+2H2O
2OH-+Cl2��+H2�����ʴ�Ϊ��2Cl-+2H2O![]() 2OH-+Cl2��+H2����
2OH-+Cl2��+H2����
(2)��ͼ��֪��2LiBH4(s)+MgH2(s)=2LiH(s)+2B(s)+MgH2(s)+3H2(g) ��H=+200 kJmol-1�٣�2LiBH4(s)+MgH2(s) =2LiBH4(s)+Mg(s)+H2(g) ��H=+76 kJmol-1�ڣ�2LiBH4(s)+MgH2(s) =2LiH(s)+MgB2(s)+4H2(g) ��H=+183 kJmol-1�ۣ����ݢٵõ�2LiH(s)+2B(s)+3H2(g) �T2LiBH4(s) ��H=-200kJmol-1���ʴ�Ϊ��2LiH(s)+2B(s)+3H2(g) �T2LiBH4(s) ��H=-200kJmol-1��
��(3)��ʯī�������缫���ŨNaOH��Һ�Ʊ���������(Na2FeO4)����ʧ�������ɸ������ƣ�������������ʯī������������A�ǵ�Դ��������B�Ǹ�����������ý��������������������ԭ�Ե��������ӣ����ܻ�ø���������ӣ������缫���ܻ���ʯī�缫���ʴ�Ϊ�����������ܣ�
(4)����������ʯī���������������Ϸ����õ��ӵĻ�ԭ��Ӧ����Һ�е������ӷŵ磬��2H++2e-=H2����2H2O+2e-=H2��+2OH-���ʴ�Ϊ��2H++2e-=H2����2H2O+2e-=H2��+2OH-��
(5)��ػ����ɺ��ɫ���������������Ӵ����ǿ�ȣ��Ϳ���ʹ���ɫ����������⣬ת��ΪFeO42-��������Ӧ��Fe(OH)3-3e-+5OH-�TFeO42-+4H2O���ʴ�Ϊ��Fe(OH)3-3e-+5OH-�TFeO42-+4H2O��
(6)��Ϊԭ��أ�ͨ��״��ĵ缫Ϊ������ͨ�������ĵ缫Ϊ��������ACΪ������BDΪ����������B�缫Ϊ��������Һ��ͭ��������������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��˵��ͭ���ӷŵ����Һ�е������ӵõ����������������������������ʵ���Ϊx����Һ��ͭ�������ʵ���Ϊ0.1mol��Cu2++2e-=Cu��ת�Ƶ���0.2mol��2H++2e-=H2����ת�Ƶ���2x����������ת�Ƶ���0.2 mol +2x��A�缫Ϊ��������Һ�е�����������ʧ��������������4OH--4e-=2H2O+O2����ת�Ƶ���4x�����ݵ�ʧ�����غ�õ���0.2mol+2x=4x����ã�x=0.1mol����״���£��������������V=nVm=0.1mol��22.4L/mol=2.24L���ʴ�Ϊ��������2.24L��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ���������⡿����ȩ������ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶȣ��ڼ��������·����绯��Ӧ�����Ʊ�����ȩ����ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶȣ��������ᡣ��Ӧԭ�����£�
2C6H5CHO+NaOH![]() C6H5CH2OH+C6H5COONa
C6H5CH2OH+C6H5COONa
C6H5COONa+HCl![]() C6H5COOH+NaCl
C6H5COOH+NaCl
������������������±���
����ȩ | ���״� | ������ | �� | |
�е�/�� | 178 | 205 | 249 | 80 |
�۵�/�� | 26 | -15 | 122 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ���������£�
��1���ڢ�����������1Сʱ����ͼ1�������м��Ȼ�Ϲ̶�װ��Ϊ������
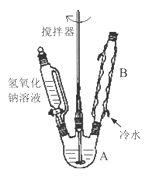
����A������Ϊ_______����������B��Ϊ����C��Ч������B��˵��ԭ��_______��
��2���������йط�Һ©����ʹ�ò���ȷ����_______
A.��Һ©����ʹ��֮ǰ��������Ƿ�©ˮ
B.��Һ©���ڵ�Һ�岻�ܹ��࣬����������
C.�����Һ©����������̨�Ͼ��ã��ֲ���������������з�Һ
D.��Һʱ���²�Һ�����������ر������������ձ��ٴ�����ʹ�ϲ�Һ������
��3�����������÷�ˮԡ���������ٽ��в����ܣ���ͼ2�����ռ�______�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ_____________��
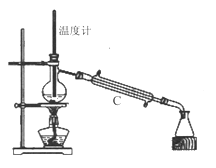
��4������ʱ����ͼ3���ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��_____��ϴ�����ϲ����ľ��塣������ɺ���������ˮ�Ծ������ϴ�ӣ�ϴ��Ӧ____________��
��5���õ�����ƽȷ��ȡ0.2440g����������ƿ�м�100mL����ˮ�ܽ⣨��Ҫʱ���Լ��ȣ�������0.1000mol/L�ı�����������Һ�ζ��������ı�����������Һ19.20mL��������Ĵ���Ϊ_____%��


