��Ŀ����
 ��1����Ҫ����500mL 0.200mol?L-1��H2SO4��Һ����Ҫ����������Ͳ���ձ����______��
��1����Ҫ����500mL 0.200mol?L-1��H2SO4��Һ����Ҫ����������Ͳ���ձ����______��
��Ҫ98%��Ũ���ᣨ�ܶ�1.84g?cm-3��______mL��
�����в�����ʹ�������ҺŨ��ƫС����______������ţ���
A������Ͳȡһ�������98%��Ũ���ᣬϡ�ͺ�δ����ȴ��ת������ƿ��
B��ϡ���������õ�С�ձ�δϴ��
C������ʱ���ӹ۲�Һ��
D��������ˮϴ�Ӻ������ƿδ����
E������ҡ�Ⱥ��������Լ�ƿ�д���ʱ����������Һ����ƿ��
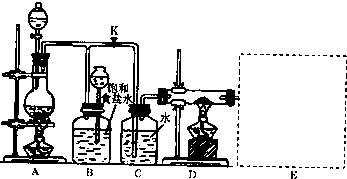
��2����ͼ��һ��ʵ�����Ƶ�������������Ϊԭ�Ͻ����ض���Ӧ��װ��
��A����������װ�ã����з��������ӷ���ʽΪ______��
��ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼD���ƾ��ƣ�Cl2ͨ��Cƿ���ٽ���D��Dװ�õ�Ӳ�ʲ�������ʢ��̿�ۣ�����������ԭ��Ӧ�������ΪCO2��HCl����÷�Ӧ�Ļ�ѧ����ʽΪ______��
�۸�װ��ͼ��δ���꣬����E��������������ע���Լ����ƣ�
��D����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ����������ȵ����ã�A������Cl2��������B��������______��
����ʵ����ʹ��12mol?L-1�� Ũ����10mL��������MnO2��Ӧ�������ɵ�Cl2�����ʵ�������С��0.03mol���Է������ܴ��ڵ�ԭ����______��
�⣺��1�����Ʋ����У����㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ�Ȳ�����һ������Ͳ��ȡ�����轺ͷ�ιܣ������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ�Ӳ���ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ����ҡ�Ⱥ�װƿ����ǩ��������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ���Ͳ��500mL����ƿ��
�ʻ���Ҫ�������У���������500mL����ƿ����ͷ�ιܣ�
����Ҫ98%H2SO4�����ΪVmL��������Һϡ��ǰ����������������VmL��1.84g/cm3��98%=0.5L��0.2mol?L-1��98g/mol�����V=5.4ml��
�ʴ�Ϊ����������500mL����ƿ����ͷ�ιܣ�5.4mL��
��A������Ͳ��ȡһ�������98%��Ũ���ᣬϡ�ͺ�δ����ȴ��ת������ƿ�ڣ�����Һ�����ƫ��һ����ȴ��������Һ�����ƫС��Ũ��ƫ��A�����ϣ�
B��ϡ���������õ�С�ձ�δϴ�ӣ����ձ��ڱ�մ�����ʣ��������ʵ�����ƫС��Ũ��ƫС����B���ϣ�
C������ʱ���ӹ۲�Һ�棬��Һ��Һ����ڿ̶��ߣ���Һ�����ƫС��Ũ��ƫ��C�����ϣ�
D��������ˮϴ�Ӻ������ƿδ�������Һ�������Ӱ�죬Ũ�Ȳ��䣬��D�����ϣ�
E������ҡ�Ⱥ��������Լ�ƿ�д���ʱ����������Һ����ƿ�⣬�����Ǿ��ȵ���Һ������Һ��Ũ����Ӱ�죬��E�����ϣ�
�ʴ�Ϊ��B��
��2����A�з�Ӧ���Ƚ��У�Ӧ�Ƕ���������Ũ���ᷴӦ�����Ȼ��̡�������ˮ����Ӧ���ӷ���ʽΪ��MnO2+4H++2Cl-�TMn2++Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl-�TMn2++Cl2��+2H2O��
�ڼ���Dװ�õ�������������ˮ��������̼�ڼ��������·�Ӧ���ɶ�����̼��HCl����Ӧ����ʽΪ��2Cl2+2H2O+C CO2+4HCl��
CO2+4HCl��
�ʴ�Ϊ��2Cl2+2H2O+C CO2+4HCl��
CO2+4HCl��
��β���к���δ��Ӧ��������ֱ���ŷŻ���Ⱦ������Eװ������������������������Һ�������õ�©����ֹ��������E����װ��Ϊ�� ��
��
�ʴ�Ϊ��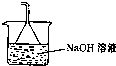 ��
��
��A������Cl2��������B�������Ǵ���������
�ʴ�Ϊ������������
���淴Ӧ����HCl���ġ���HCl�Ļӷ��������ϡ��MnO2����Ӧ�������ɵ�Cl2�����ʵ�������С��0.03mol��
�ʴ�Ϊ���淴Ӧ����HCl���ġ���HCl�Ļӷ��������ϡ��MnO2����Ӧ��
��������1���ٸ���������Һ��ʵ���������ѡ�������������
�������Һ�����������m=��V���������������Һϡ��ǰ������������������ʽ���
�ڷ������������ʵ����ʵ��������Һ�������Ӱ�죬����c=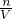 �����жϣ�
�����жϣ�
��2����A�з�Ӧ���Ƚ��У�Ӧ�Ƕ���������Ũ���ᷴӦ�����Ȼ��̡�������ˮ��
�ڼ���Dװ�õ�������������ˮ��������̼�ڼ��������·�Ӧ���ɶ�����̼��HCl��
��β���к���δ��Ӧ��������ֱ���ŷŻ���Ⱦ������Eװ������������������������Һ�������õ�©����ֹ������
��A������Cl2��������B�������Ǵ���������
��HCl�Ļӷ������ᷴӦ�������ϡ��MnO2����Ӧ��
���������⿼��һ�����ʵ���Ũ����Һ�����ơ�������ʵ�����Ʊ��ȣ��Ѷ��еȣ���Ҫѧ��������ʵ�Ļ���֪ʶ������֪ʶ������������������
�ʻ���Ҫ�������У���������500mL����ƿ����ͷ�ιܣ�
����Ҫ98%H2SO4�����ΪVmL��������Һϡ��ǰ����������������VmL��1.84g/cm3��98%=0.5L��0.2mol?L-1��98g/mol�����V=5.4ml��
�ʴ�Ϊ����������500mL����ƿ����ͷ�ιܣ�5.4mL��
��A������Ͳ��ȡһ�������98%��Ũ���ᣬϡ�ͺ�δ����ȴ��ת������ƿ�ڣ�����Һ�����ƫ��һ����ȴ��������Һ�����ƫС��Ũ��ƫ��A�����ϣ�
B��ϡ���������õ�С�ձ�δϴ�ӣ����ձ��ڱ�մ�����ʣ��������ʵ�����ƫС��Ũ��ƫС����B���ϣ�
C������ʱ���ӹ۲�Һ�棬��Һ��Һ����ڿ̶��ߣ���Һ�����ƫС��Ũ��ƫ��C�����ϣ�
D��������ˮϴ�Ӻ������ƿδ�������Һ�������Ӱ�죬Ũ�Ȳ��䣬��D�����ϣ�
E������ҡ�Ⱥ��������Լ�ƿ�д���ʱ����������Һ����ƿ�⣬�����Ǿ��ȵ���Һ������Һ��Ũ����Ӱ�죬��E�����ϣ�
�ʴ�Ϊ��B��
��2����A�з�Ӧ���Ƚ��У�Ӧ�Ƕ���������Ũ���ᷴӦ�����Ȼ��̡�������ˮ����Ӧ���ӷ���ʽΪ��MnO2+4H++2Cl-�TMn2++Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl-�TMn2++Cl2��+2H2O��
�ڼ���Dװ�õ�������������ˮ��������̼�ڼ��������·�Ӧ���ɶ�����̼��HCl����Ӧ����ʽΪ��2Cl2+2H2O+C
 CO2+4HCl��
CO2+4HCl���ʴ�Ϊ��2Cl2+2H2O+C
 CO2+4HCl��
CO2+4HCl����β���к���δ��Ӧ��������ֱ���ŷŻ���Ⱦ������Eװ������������������������Һ�������õ�©����ֹ��������E����װ��Ϊ��
 ��
���ʴ�Ϊ��
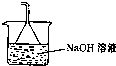 ��
����A������Cl2��������B�������Ǵ���������
�ʴ�Ϊ������������
���淴Ӧ����HCl���ġ���HCl�Ļӷ��������ϡ��MnO2����Ӧ�������ɵ�Cl2�����ʵ�������С��0.03mol��
�ʴ�Ϊ���淴Ӧ����HCl���ġ���HCl�Ļӷ��������ϡ��MnO2����Ӧ��
��������1���ٸ���������Һ��ʵ���������ѡ�������������
�������Һ�����������m=��V���������������Һϡ��ǰ������������������ʽ���
�ڷ������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
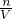 �����жϣ�
�����жϣ���2����A�з�Ӧ���Ƚ��У�Ӧ�Ƕ���������Ũ���ᷴӦ�����Ȼ��̡�������ˮ��
�ڼ���Dװ�õ�������������ˮ��������̼�ڼ��������·�Ӧ���ɶ�����̼��HCl��
��β���к���δ��Ӧ��������ֱ���ŷŻ���Ⱦ������Eװ������������������������Һ�������õ�©����ֹ������
��A������Cl2��������B�������Ǵ���������
��HCl�Ļӷ������ᷴӦ�������ϡ��MnO2����Ӧ��
���������⿼��һ�����ʵ���Ũ����Һ�����ơ�������ʵ�����Ʊ��ȣ��Ѷ��еȣ���Ҫѧ��������ʵ�Ļ���֪ʶ������֪ʶ������������������

��ϰ��ϵ�д�
�����Ŀ
 A
A  B
B 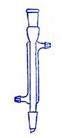 C
C  D
D