��Ŀ����
��1����֪�ڳ����²��Ũ�Ⱦ�Ϊ0��1 mol��L-1������6����Һ��pHֵ
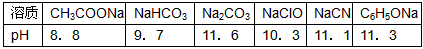
___________�����ţ���
A��CO2+H2O +2NaClO = Na2CO3+2HClO
B��CO2+H2O +NaClO = NaHCO3+HClO
C��CO2 +H2O +C6H5ONa
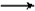 NaHCO3+C6H5OH
NaHCO3+C6H5OH D��CO2 +H2O +2C6H5ONa
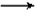 Na2CO3+2C6H5OH
Na2CO3+2C6H5OHE��Na2CO3+C6H5OH
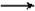 NaHCO3+C6H5ONa
NaHCO3+C6H5ONa F��CH3COOH+NaCN = CH3COONa+HCN
��2������ǰ����Ϣ�жϣ������£�Ũ�Ⱦ�Ϊ0��05 mol��L-1������5�����ʵ���Һ�У�pH��С����______
(����)����pHֵΪ_______(����ֵ)��pH������_______�����ţ���
��HCN ��CH3COOH ��HClO4 ��HClO ��H2SO4
��3��һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��������������Ӧ���ܽ�����ֽⷴӦ��������һ���ɣ�____________________________��
��4������(3)�н��ۣ��ֽ�KI��Һ��AgCl�����Ͻ��裬����ܻ�۲쵽��������______________����Ӧ�����ӷ���ʽΪ_____________________��
��2���ݣ�1����
��3�����ֽⷴӦ�ܹ������ɸ����ܣ��ܽ�ȸ�С�����ʵķ������
��4����ɫ�����ɻ�ɫ��I-+AgCl==AgI+Cl-

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
��1����֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1mol/L������6����Һ��pHֵ��
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | NaR |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3===2CH3COONa+CO2��+H2O �������Ƕȿ�����ͬʱ��ʾ����һ�����ɣ������Խ�ǿ�����ʷ������Ʒ�Ӧ�������ɼ��Խ��������ʡ����ոù��ɣ����ж����з�Ӧ���ܳ�������_______________________�����ţ���
��A��CO2+H2O +2NaClO===Na2CO3+2HClO
��B��CO2+H2O +NaClO===NaHCO3+HClO
��C��CO2 +H2O +NaR![]() NaHCO3+HR
NaHCO3+HR
��D��CO2 +H2O +2NaR![]() Na2CO3+2HR
Na2CO3+2HR
��E��Na2CO3+HR![]() NaHCO3+NaR
NaHCO3+NaR
��F��CH3COOH+NaCN===CH3COONa+HCN
��2������ǰ����Ϣ�жϣ�Ũ�Ⱦ�Ϊ0.05 mol/L�������������ʵ���Һ�У�pH��С����______(����)�� ��pHΪ___________(����ֵ)��pH������_________�����ţ���
��HR ��CH3COOH ��HCN ��HClO ��H2SO4 ��HClO4
��3��һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ���
������KCl��NaNO3�����Һ����������NaCl����
��������������Ӧ���ܽ�����ֽⷴӦ��������һ���ɣ�______________________��
������KI��Һ��AgCl�����Ͻ��裬��۲쵽��������_______________________��
������д����Ӧ�����ӷ���ʽ��____________________________________________��
(��10��)���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol��L��1������6����Һ��pHֵ��
|
���� |
CH3COONa |
NaHCO3 |
Na2CO3 |
NaClO |
NaCN |
C6H5ONa |
|
pH |
8.8 |
9.7 |
11.6 |
10.3 |
11.1 |
11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3===2CH3COONa+CO2��+H2O�����ոù��ɣ����ж����з�Ӧ���ܳ�������______�����ţ���
A��CO2+H2O +2NaClO===Na2CO3+2HClO����������B��CH3COOH+NaCN===CH3COONa+HCN
C��CO2 +H2O +C6H5ONa��NaHCO3+C6H5OH ����D��CO2 +H2O +2C6H5ONa��Na2CO3+2C6H5OH
��2������ǰ����Ϣ�жϣ������£�Ũ�Ⱦ�Ϊ0.05 mol��L��1������5�����ʵ���Һ�У�pH��С���� (����)����pHֵԼΪ_______(����ֵ)��
��HCN ��CH3COOH ��HClO4 ��HClO ��H2SO4
(3)һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ���ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��塣
�����������Ӧ���ܽ�����ֽⷴӦ��������һ���� ����������ۣ��ֽ�Na2S��AgI�����Ͻ��裬��Ӧ�����ӷ���ʽ ������������ ��
���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol��L-1������6����Һ��pHֵ��
|
���� |
CH3COONa |
NaHCO3 |
Na2CO3 |
NaClO |
NaCN |
C6H5ONa |
|
pH |
8.8 |
9.7 |
11.6 |
10.3 |
11.1 |
11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3 = 2CH3COONa+CO2��+H2O ���ոù��ɣ����ж����з�Ӧ���ܳ�������___________�����ţ���
A��CO2+H2O +2NaClO = Na2CO3+2HClO
B��CO2+H2O +NaClO = NaHCO3+HClO
C��CO2 +H2O +C6H5ONa NaHCO3+C6H5OH
NaHCO3+C6H5OH
D��CO2 +H2O +2C6H5ONa Na2CO3+2C6H5OH
Na2CO3+2C6H5OH
E��Na2CO3+C6H5OH NaHCO3+C6H5ONa
NaHCO3+C6H5ONa
F��CH3COOH+NaCN = CH3COONa+HCN
(2)һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��塣�����������Ӧ���ܽ�����ֽⷴӦ��������һ���� ��
��������ۣ��ֽ�Na2S��AgI�����Ͻ��裬��Ӧ�����ӷ���ʽ ��
(7��)���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
��1����֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1mol/L������6����Һ��pHֵ��
|
���� |
CH3COONa |
NaHCO3 |
Na2CO3 |
NaClO |
NaCN |
C6H5ONa |
|
pH |
8.8 |
9.7 |
11.6 |
10.3 |
11.1 |
11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3===2CH3COONa+CO2��+H2O �������Ƕȿ�����ͬʱ��ʾ����һ�����ɣ������Խ�ǿ�����ʷ������Ʒ�Ӧ�������ɼ��Խ��������ʡ����ոù��ɣ����ж����з�Ӧ���ܳ�������_______________________�����ţ���
A��CO2+H2O +2NaClO=Na2CO3+2HClO
B��CO2+H2O +NaClO=NaHCO3+HClO
C��CO2 +H2O +C6H5ONa NaHCO3+C6H5OH
NaHCO3+C6H5OH
D��CO2 +H2O +2C6H5ONa Na2CO3+2C6H5OH
Na2CO3+2C6H5OH
E��Na2CO3+C6H5OH NaHCO3+C6H5Ona
NaHCO3+C6H5Ona
F��CH3COOH+NaCN=CH3COONa+HCN
��2������ǰ����Ϣ�жϣ�Ũ�Ⱦ�Ϊ0.05 mol/L�������������ʵ���Һ�У�pH��С����______(����)����pHΪ___________(����ֵ)��pH������_________�����ţ���
��C6H5OH ��CH3COOH ��HCN ��HClO ��H2SO4 ��HClO4
��3��һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ���
������KCl��NaNO3�����Һ����������NaCl�������������Ӧ���ܽ�����ֽⷴӦ��������һ���ɣ� ��
��KI��Һ��AgCl�����Ͻ��裬��۲쵽�������� ��
��д����Ӧ�����ӷ���ʽ�� ��