��Ŀ����
��ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪��
��CO��g��+
O2��g��=CO2��g����H=-283.0kJ/mol
��H2��g��+
O2��g��=H2O��l����H=-285.8kJ/mol
��CH3OH��g��+
O2��g��=2H2O��l��+CO2��g����H=-761.7kJ/mol
��CO��g��+2H2��g��=CH3OH��g��
��1����Ӧ�ܵġ�H=______����S______���������������=������
��2����һ�������£���Ӧ����һ�ܱ������дﵽƽ�⣮ά��H2Ũ�Ⱥ��������¶Ȳ��䣬���������������ƽ�⽫______������ĸ����
A��������Ӧ�����ƶ� B�����淴Ӧ�����ƶ�
C�����ƶ� D�����ж�
��3����ҵ���÷�Ӧ�ܵ�ѹ�ϳɼ״�����230�桫270����Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱȣ��ֱ���230�桢250���270�����ʵ�飬�����ͼ��230���ʵ��������Ӧ��������______������ĸ������ҵ�������˲��õĺϳ�����ɱ�n��H2����n��CO���ķ�ΧӦ��______��������ĸ��
A��1��1��1.5��1 B��2.2��1��3��1 C��3.5��1��4.5��1
��4��ԭ�����к�������CO2��CO�ϳɼ״���ת������һ��Ӱ�죮��ѧ��Ϊ�о���һӰ�죬��ͬһ�����зֱ��������5��ʵ�飮
����5��ʵ���У����Ʋ����ʵ��������ѹǿ��������______��______�ȣ�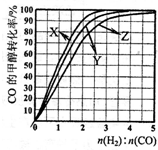
��CO��g��+
| 1 |
| 2 |
��H2��g��+
| 1 |
| 2 |
��CH3OH��g��+
| 3 |
| 2 |
��CO��g��+2H2��g��=CH3OH��g��
��1����Ӧ�ܵġ�H=______����S______���������������=������
��2����һ�������£���Ӧ����һ�ܱ������дﵽƽ�⣮ά��H2Ũ�Ⱥ��������¶Ȳ��䣬���������������ƽ�⽫______������ĸ����
A��������Ӧ�����ƶ� B�����淴Ӧ�����ƶ�
C�����ƶ� D�����ж�
��3����ҵ���÷�Ӧ�ܵ�ѹ�ϳɼ״�����230�桫270����Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱȣ��ֱ���230�桢250���270�����ʵ�飬�����ͼ��230���ʵ��������Ӧ��������______������ĸ������ҵ�������˲��õĺϳ�����ɱ�n��H2����n��CO���ķ�ΧӦ��______��������ĸ��
A��1��1��1.5��1 B��2.2��1��3��1 C��3.5��1��4.5��1
��4��ԭ�����к�������CO2��CO�ϳɼ״���ת������һ��Ӱ�죮��ѧ��Ϊ�о���һӰ�죬��ͬһ�����зֱ��������5��ʵ�飮
| ��� | ԭ�����и���ֵ�������� | |||
| CO | CO2 | H2 | N2 | |
| ��1�� | 19.7 | 0.0 | 59.1 | 21.2 |
| ��2�� | 20.7 | 0.3 | 62.1 | 16.9 |
| ��3�� | 16.9 | 1.3 | 50.7 | 31.1 |
| ��4�� | 19.8 | 5.5 | 59.4 | 15.3 |
| ��5�� | 20.3 | 10.9 | 60.9 | 7.9 |

��1����Ӧ��CO��g��+2H2��g��=CH3OH��g������Ӧǰ������������ٵķ�Ӧ���ر����С��0�����ݸ�˹���ɵõ���
��+2����-�ۣ��ܵ��Ȼ�ѧ����ʽΪ��
CO��g��+2H2��g��=CH3OH��g����H=-92.9KJ/mol
�ʴ�Ϊ����H��0����S��0��
��2����һ�������£���Ӧ����һ�ܱ������дﵽƽ�⣮ά��H2Ũ�Ⱥ��������¶Ȳ��䣬���������������ѹǿ��С��ƽ��������У��ʴ�Ϊ��B��
��3����ҵ���÷�Ӧ�ܵ�ѹ�ϳɼ״�����230�桫270����Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱȣ��ֱ���230�桢250���270�����ʵ�飬�����ͼ���ϳɼ״��Ƿ��ȷ�Ӧ���¶�Խ��ת����Խ���ͼ���֪��230���ʵ��������Ӧ�������� X������ҵ�������˲��õĺϳ�����ɱ�n��H2����n��CO���ķ�ΧӦ��Ӧ�� һ����̼��ת���ʴ�����������������һ����̼��ת���ʣ�����n��H2����n��CO����2��1��ѡ����������������С��ԭ�ϣ���ѡB��
�ʴ�Ϊ��X��B��
��4������5��ʵ���У��������ݷ����жϣ����Ʋ����ʵ��������ѹǿ���������¶ȡ�CO��H2���������֮�ȵȣ���D��Ϊ���¶ȡ�CO��H2���������֮�ȣ�
��+2����-�ۣ��ܵ��Ȼ�ѧ����ʽΪ��
CO��g��+2H2��g��=CH3OH��g����H=-92.9KJ/mol
�ʴ�Ϊ����H��0����S��0��
��2����һ�������£���Ӧ����һ�ܱ������дﵽƽ�⣮ά��H2Ũ�Ⱥ��������¶Ȳ��䣬���������������ѹǿ��С��ƽ��������У��ʴ�Ϊ��B��
��3����ҵ���÷�Ӧ�ܵ�ѹ�ϳɼ״�����230�桫270����Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱȣ��ֱ���230�桢250���270�����ʵ�飬�����ͼ���ϳɼ״��Ƿ��ȷ�Ӧ���¶�Խ��ת����Խ���ͼ���֪��230���ʵ��������Ӧ�������� X������ҵ�������˲��õĺϳ�����ɱ�n��H2����n��CO���ķ�ΧӦ��Ӧ�� һ����̼��ת���ʴ�����������������һ����̼��ת���ʣ�����n��H2����n��CO����2��1��ѡ����������������С��ԭ�ϣ���ѡB��
�ʴ�Ϊ��X��B��
��4������5��ʵ���У��������ݷ����жϣ����Ʋ����ʵ��������ѹǿ���������¶ȡ�CO��H2���������֮�ȵȣ���D��Ϊ���¶ȡ�CO��H2���������֮�ȣ�

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
��ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״�����֪��CO��g��+2H2��g��?CH3OH��g����H=-92.9kJ/mol��һ�������£��÷�Ӧ��һ����̶����ܱ������дﵽƽ�⣮����˵����ȷ���ǣ�������
| A���÷�Ӧ�ġ�S��0 | B���÷�Ӧ���κ��¶��¾����Է����� | C���������г�������He��ƽ��������Ӧ�����ƶ� | D�������¶�ƽ��������Ӧ�����ƶ� |
 ��2009?�Ͼ���ģ����ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪��
��2009?�Ͼ���ģ����ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪��
 CH3OH(g)
��H=��92.9kJ/mo1
CH3OH(g)
��H=��92.9kJ/mo1