��Ŀ����
��ʽ̼��ͭ[xCuCO3��yCu(OH)2]���ʿ�ȸ����ɫ.�ֳ�Ϊ��ȸʯ����һ������Ŀ��ﱦʯ������ͭ������е�������������̼��ˮ���������ʷ�Ӧ���������ʡ�CuSO4��Һ��Na2CO3��Һ��Ӧ���Եõ���ʽ̼��ͭ�����ǽ�������ɽ������̽����
[�����Ʊ�]
��ȡ12. 5 g����(CuSO4• 5H2O)����87. 5mL����ˮ�У��μ�����ϡ����(������Ժ��Բ���)����ֽ����õ�CuSO4��Һ�������м���Na2CO3��Һ������������ɫ����Һ���ˣ���������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ����͜غ�ɱ��á�
[ʵ��̽��]�������������װ�ã����Ƶõ�����ɫ�������ʵ�顣
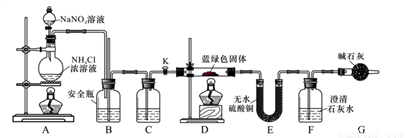
��������ʵ��ش���������
(1)��������ͭ��Һ�Ĺ����еμ�ϡ�����������___________����������ͭ��Һ��������������Ϊ_________
(2)ʵ����ͨ��ʹ�ü����������ƺ��Ȼ�炙����Һ�ķ�����ȡN2���÷�Ӧ�Ļ�ѧ����Ϊ��__________��
��Dװ�ü���ǰ����Ҫ���ȴ���K��ͨ������N2��Ȼ��ر�K���ٵ�ȼD���ƾ��ơ�ͨ��N2����
��___________�� BΪ��ȫƿ��������ԭ��Ϊ_________��C��ʢװ���Լ�Ӧ��__________��
(4)����D��۲쵽��������________________��
(5)����������֪��Ksp[CaCO3]=2.8��10-9��Ksp[BaCO3]=5.1��10-9����������Ϊ��Ҫ��Ba(OH)2��Һ�������ʯ��ˮ�������ⶨ����ɫ����Ļ�ѧʽ����ԭ����______________
a.Ba(OH)2�ļ��Ա�Ca(OH)2ǿ
b.Ba(OH)2�ܽ�ȴ���Ca(OH)2���ܳ������CO2
c.��ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
d.���յ���CO2���ɵ�BaCO3����������CaCO3���������С
(6)��D�з�Ӧ��ȫ����K���ٴεμ�NaNO2��Һ����N2����Ŀ����______________����װ��F��ʹ��Ba(OH)2��Һ��ʵ�����������װ��E����������0.27 g��F�в�������1.97 g���������ɫ����Ļ�ѧʽΪ_____________��[д��xCuCO3��yCu(OH)2����ʽ]


 2I����S4O62��)������14.00 mL��
2I����S4O62��)������14.00 mL�� 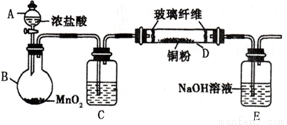

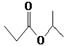 D. ��ȩ�Ľṹ��ʽ��CH3CH2COH
D. ��ȩ�Ľṹ��ʽ��CH3CH2COH