��Ŀ����
��15��)ij�о���ѧϰС�齫һ��Ũ��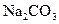 ��Һ����
��Һ���� ��Һ�еõ���ɫ������
��Һ�еõ���ɫ������
��ͬѧ��Ϊ���߷�Ӧ����ֻ�� һ�ֳ�����
һ�ֳ�����
��ͬѧ��Ϊ��������ٽ�ˮ�ⷴӦ������ һ�ֳ�����
һ�ֳ�����
��ͬѧ��Ϊ���� ��
�� ���ֳ�����
���ֳ�����
(��������֪�� ��
�� �������ᾧˮ)
�������ᾧˮ)
I��������ͬѧ������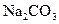 ��Һ��
��Һ�� ��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ�ٹ��ˢ�ϴ�Ӣ۸��
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
(1)��װ������˳��Ϊ �� �� ��
(2)װ��C��װ���Լ��������� ��
(3)��֤������������ ��ʵ�������� ��
��ʵ�������� ��
���� ��
�� ���߶��У���ͨ��������ʾװ�õ����ӣ����ж����������ⶨ����ɡ�
���߶��У���ͨ��������ʾװ�õ����ӣ����ж����������ⶨ����ɡ�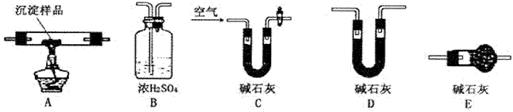
(1)װ��C�м�ʯ�ҵ������� ��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ŀ��������÷ֱ��� ��
(2)��������Ʒ������Ϊm�ˣ�װ��B����������n�ˣ�������� ����������Ϊ�� ��
������������ ��
��Na2CO3 +CuSO4 +H2O=Cu(OH)2��+Na2SO4+CO2����2�֣���
��1��A��C��B��2�֣���2����ˮ����ͭ��2�֣���3��װ��B�г���ʯ��ˮ����ǣ�2�֣�
��1�����տ����е�H2O ������CO2��2�֣�����ʼʱͨ�봦�����Ŀ������Խ�װ����ԭ�к�H2O ������CO2�Ŀ����ų�������ʱͨ�봦�����Ŀ������Խ�װ����������H2O ������CO2�ų�����2�֣�
����2��1����49n/9m����3�֣�
����



