��Ŀ����
��֪��25��ʱ��A�����ҺpH��a��B�����ҺpH��b��
(1)����AΪǿ�ᣬBΪǿ���a��b��14�����ߵ������Ϻ���Һ��pH��________����ʱ����Һ��������Ũ�ȴ���������Ũ�ȣ���ԭ�������________��ǿ��ǿ������Ϊ1��10��Ϻ���Һ�����ԣ���a��b��________��
(2)����A�Ļ�ѧʽΪHR��B�Ļ�ѧʽΪMOH����a��b��14�����ߵ������Ϻ���Һ�����ԣ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽΪ________���˻����Һ�У�������Ũ�ȴ�С��ϵһ����ȷ����________(�����)��
��c(MOH)��c(M+)��c(R��)��c(H+)��c(OH��)
��c(HR)��c(M+)��c(R��)��c(OH��)��c(H+)
��c(R��)��c(M+)��c(H+)��c(OH��)
��c(M+)��c(R��)��c(OH��)��c(H+)
��c(M+)��c(H+)��c(R��)��c(OH��)
�𰸣�
������
������
|
����(1)7(2��)����Ϊ��Ԫ(ǿ)�ᣬ��ΪһԪ(ǿ)��(2��)��13(3��) ����(2)R����H2O |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
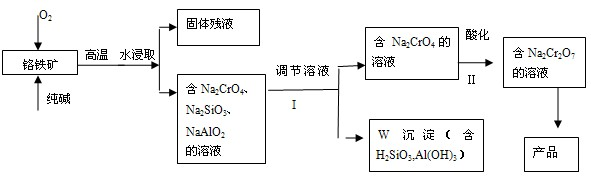
 ��ش��������⡣
��ش��������⡣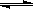 Cr2O72-
(�Ⱥ�ɫ)+H2O
Cr2O72-
(�Ⱥ�ɫ)+H2O