��Ŀ����
������H��һ�����ϣ������ڽ����У���������·�ߺϳɣ�
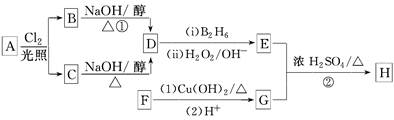
��֪��R��CH===CH2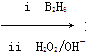 RCH2CH2OH(����B2H6Ϊ������)
RCH2CH2OH(����B2H6������)
��ش��������⣺
(1)11.2 L(��״��)����A�������г��ȼ�տ�������88 g CO2��45 g H2O��A�ķ���ʽ��________________________________________��
(2)B��C��Ϊһ�ȴ��������ǵ�����(ϵͳ����)�ֱ�Ϊ___________��
(3)�ڴ���������1 mol F��2 mol H2��Ӧ������3����1������F�Ľṹ��ʽ��___________________________________��
(4)��Ӧ�ٵķ�Ӧ������_____________________________��
(5)��Ӧ�ڵĻ�ѧ����ʽΪ__________________________��
(6)��G������ͬ�����ŵ�G�ķ�����ͬ���칹�干�����֣��������ֱַ���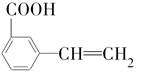 ��
��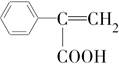 ���������ֵĽṹ��ʽ�ֱ���___________________________________��
���������ֵĽṹ��ʽ�ֱ���___________________________________��

��֪��R��CH===CH2
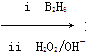 RCH2CH2OH(����B2H6������)
RCH2CH2OH(����B2H6Ϊ������)��ش��������⣺
(1)11.2 L(��״��)����A�������г��ȼ�տ�������88 g CO2��45 g H2O��A�ķ���ʽ��________________________________________��
(2)B��C��Ϊһ�ȴ��������ǵ�����(ϵͳ����)�ֱ�Ϊ___________��
(3)�ڴ���������1 mol F��2 mol H2��Ӧ������3����1������F�Ľṹ��ʽ��___________________________________��
(4)��Ӧ�ٵķ�Ӧ������_____________________________��
(5)��Ӧ�ڵĻ�ѧ����ʽΪ__________________________��
(6)��G������ͬ�����ŵ�G�ķ�����ͬ���칹�干�����֣��������ֱַ���
 ��
�� ���������ֵĽṹ��ʽ�ֱ���___________________________________��
���������ֵĽṹ��ʽ�ֱ���___________________________________��(1)C4H10��(2)2��1�ȱ��顢2��2�ȱ���
(3) ��(4)��ȥ��Ӧ
��(4)��ȥ��Ӧ
(5)


(6)

(3)
 ��(4)��ȥ��Ӧ
��(4)��ȥ��Ӧ(5)


(6)

(1)0.5 mol��A�к��е�̼ԭ�ӵ����ʵ���Ϊ88 g��44 g��mol��1��2 mol����ԭ�ӵ����ʵ���Ϊ2��45 g��18 g��mol��1��5 mol������A�ķ���ʽΪC4H10��(2)B��C���ܷ�����ȥ��Ӧ������ͬ�����ʣ���AΪ2�����飬B��C������Ϊ2��1�ȱ����2��2�ȱ��顣(3)��Ͽ�ͼ��F����G��������֪F�к���ȩ�������1 mol F��2 mol H2��Ӧ����3����1������֪F�к���һ��̼̼˫�������FΪ GΪ
GΪ ��(4)�ɢٵķ�Ӧ������֪�÷�ӦΪ��ȥ��Ӧ��DΪ(CH3)2CH===CH2��(5)��ϸ�������֪��Ϣ��֪EΪ(CH3)2CH2CH2OH����Ӧ��ΪG��E������������Ӧ�����H�Ľṹ��ʽΪ
��(4)�ɢٵķ�Ӧ������֪�÷�ӦΪ��ȥ��Ӧ��DΪ(CH3)2CH===CH2��(5)��ϸ�������֪��Ϣ��֪EΪ(CH3)2CH2CH2OH����Ӧ��ΪG��E������������Ӧ�����H�Ľṹ��ʽΪ ��(6)����ȡ����λ�ڱ�������λ�Ͷ�λ����
��(6)����ȡ����λ�ڱ�������λ�Ͷ�λ����
 GΪ
GΪ ��(4)�ɢٵķ�Ӧ������֪�÷�ӦΪ��ȥ��Ӧ��DΪ(CH3)2CH===CH2��(5)��ϸ�������֪��Ϣ��֪EΪ(CH3)2CH2CH2OH����Ӧ��ΪG��E������������Ӧ�����H�Ľṹ��ʽΪ
��(4)�ɢٵķ�Ӧ������֪�÷�ӦΪ��ȥ��Ӧ��DΪ(CH3)2CH===CH2��(5)��ϸ�������֪��Ϣ��֪EΪ(CH3)2CH2CH2OH����Ӧ��ΪG��E������������Ӧ�����H�Ľṹ��ʽΪ ��(6)����ȡ����λ�ڱ�������λ�Ͷ�λ����
��(6)����ȡ����λ�ڱ�������λ�Ͷ�λ����

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ
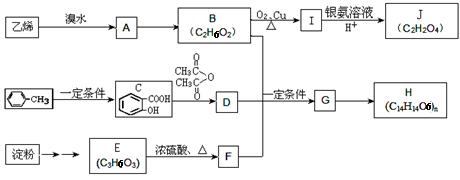
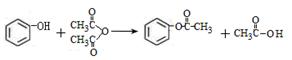
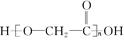 ������������
���������м��л���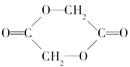 �ۺ϶��ɵġ�
�ۺ϶��ɵġ�
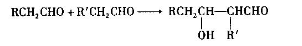

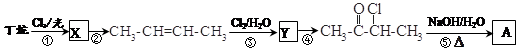
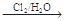 ClCH2��CH2��OH
ClCH2��CH2��OH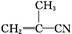
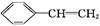

 CH��CN
CH��CN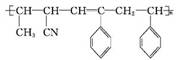 �ĸ߷��Ӳ��ϵ���ȷ���Ϊ�� ��
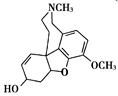
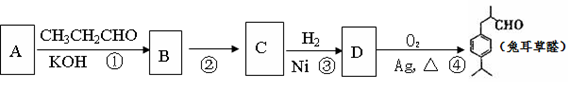
�ĸ߷��Ӳ��ϵ���ȷ���Ϊ�� ��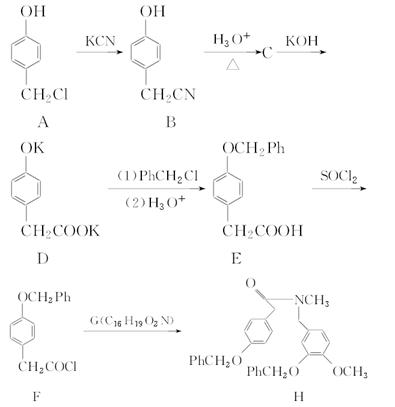
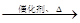 2CH3CHO
2CH3CHO 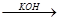 CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO