��Ŀ����
��9�֣�����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء��ش��������⣺
| | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶(10��9m) | 0.074 | 0.160 | 0.152 | 0.110 | 0.099 | 0.186 | 0.075 | 0.082 | 0.102 | 0.037 |
| ������ ���ϼ� | | ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | +6[ | +1 |
| ��2 | | | ��3 | ��1 | | ��3 | | -2 | |
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��
��3��������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ________________������Ӽ������Լ���Ǽ��Լ�����
��F2A2��J2A�ķ�Ӧ����3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2�������� g��
��4���õ���ʽ��ʾJ2A���γɹ��̣� ��
(1) B; �������ڵ�IIA�壻 ��2��HClO4;
(3) 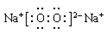 ���Ӽ����Ǽ��Լ���39g
���Ӽ����Ǽ��Լ���39g
��4��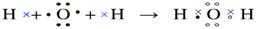
(�ڣ�4����2�֣�������ӷֱ��õ�Ͳ��ʾ�ˣ���ǰ��������Ӧ������û��)
��(3)��ڶ����ա����Ӽ����Ǽ��Լ���2�֣����һ����һ�֣�����ÿ��ÿ��1��
����

��9�֣�����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء��ش��������⣺
|
| A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶(10��9m) | 0.074 | 0.160 | 0.152 | 0.110 | 0.099 | 0.186 | 0.075 | 0.082 | 0.102 | 0.037 |
| ������ ���ϼ� |
| ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | +6[ | +1 |
| ��2 |
|
| ��3 | ��1 |
| ��3 |
| -2 |
|
��1��H��Ԫ�ط����� ; B��Ԫ�����ڱ��е�λ���ǣ����ڡ��壩
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��
��3��������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ________________������Ӽ������Լ���Ǽ��Լ�����
��F2A2��J2A�ķ�Ӧ����3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2�������� g��
��4���õ���ʽ��ʾJ2A���γɹ��̣� ��
��18�֣��ƶ��⣺
����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء�
|
| A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶(10��10m) | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 0.82 | 1.02 | 0.37 |
| �����ͻ��ϼ� |
| ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | +6 | +1 |
| ��2 |
|
| ��3 | ��1 |
| ��3 |
| -2 |
|
�ش��������⣺
��1��D��Ԫ�������� ��H��Ԫ�ط����� ��
B��Ԫ�����ڱ��е�λ���ǣ����ڡ��壩 ��
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��
������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ ��
��3���õ���ʽ��ʾA������⻯����γɹ��̣� ��G����̬�⻯��ĽṹʽΪ ��
��4��һ�������£�IA2����������������A2��ַ�Ӧ����20gIA3���壬�ų�24.6 kJ������д�����Ȼ�ѧ����ʽ ��
��18�֣��ƶ��⣺
����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء�
| | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶(10��10m) | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 0.82 | 1.02 | 0.37 |
| �����ͻ��ϼ� | | ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | +6 | +1 |
| ��2 | | | ��3 | ��1 | | ��3 | | -2 | |
��1��D��Ԫ�������� ��H��Ԫ�ط����� ��
B��Ԫ�����ڱ��е�λ���ǣ����ڡ��壩 ��
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��
������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ ��
��3���õ���ʽ��ʾA������⻯����γɹ��̣� ��G����̬�⻯��ĽṹʽΪ ��
��4��һ�������£�IA2����������������A2��ַ�Ӧ����20gIA3���壬�ų�24.6 kJ������д�����Ȼ�ѧ����ʽ ��



 2SO3��g������H=-196.8kJ/mol
2SO3��g������H=-196.8kJ/mol