��Ŀ����
ijУ��ѧС���ͬѧ��һ����������·������õ�Cu��Al��Fe������Au��Pt�Ƚ����Ļ���������������Ʊ�ǿ��ͭ������������ķ�����
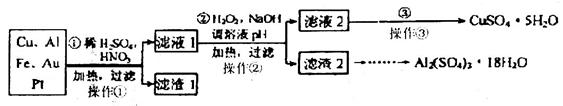
�ش��������⣺
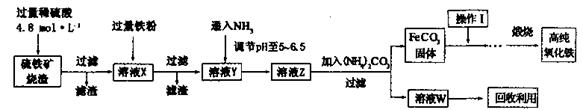
��1���ڢڲ�����H2O2��Ϊ�˳���Fe2+���÷�Ӧ�����ӷ���ʽΪ_________��
��2������2����Ҫ�ɷ���Fe(OH)3��Al(OH)3��������2��ȡAl2(SO4)3��18H2Oʵ����̵��������__________________��
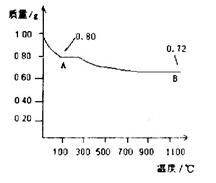
��3���õڢ۲�����CuSO4��5H2O���Ƶ�Cu(OH)2����ѧ�С��Ϊ̽��Cu(OH)2���ȷֽ���P�������ʣ��������ʵ����̣�ȡ0.98g Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ____��Cu2O��ͨ������ʵ���ͼ����Եó����½��ۣ�����ʱB______������ȶ������ȶ�������
�С��ͬѧ������������ʵ�飺
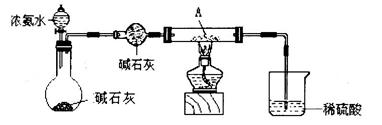
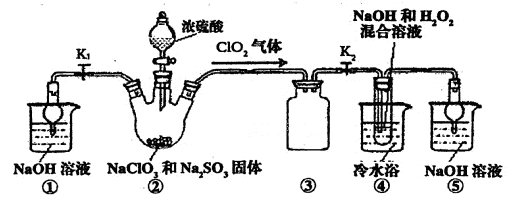
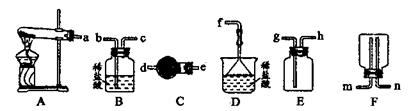
�ټ�ͬѧ������װ�ý���ʵ�飨�г�װ��ĩ�����������յó��Ľ����Ǽ���ʱA�ɽ�NH3����ΪN2��A����ԭΪ����Cu��֧�ּ�ͬѧ���۵�������______��
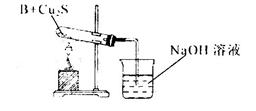
����ͬѧ������ʵ��װ�ý���ʵ�飬ʵ���й۲쵽��Ϸ�ĩ���ձ�ɺ�ɫ���ʣ�ͬʱ����һ����ɫ���д̼�����ζ�����壬�����廯ѧʽΪ_______��������Ϊ��ͬѧ��ʵ��װ������װB���ʵ��Թ����ձ�֮�����һ��������װ�ã�����Ϊ�Ƿ��б�Ҫ_____�����б�Ҫ�����ԭ���ԸĽ�____________��

�ش��������⣺
��1���ڢڲ�����H2O2��Ϊ�˳���Fe2+���÷�Ӧ�����ӷ���ʽΪ_________��
��2������2����Ҫ�ɷ���Fe(OH)3��Al(OH)3��������2��ȡAl2(SO4)3��18H2Oʵ����̵��������__________________��
��3���õڢ۲�����CuSO4��5H2O���Ƶ�Cu(OH)2����ѧ�С��Ϊ̽��Cu(OH)2���ȷֽ���P�������ʣ��������ʵ����̣�ȡ0.98g Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ____��Cu2O��ͨ������ʵ���ͼ����Եó����½��ۣ�����ʱB______������ȶ������ȶ�������

�С��ͬѧ������������ʵ�飺
�ټ�ͬѧ������װ�ý���ʵ�飨�г�װ��ĩ�����������յó��Ľ����Ǽ���ʱA�ɽ�NH3����ΪN2��A����ԭΪ����Cu��֧�ּ�ͬѧ���۵�������______��

����ͬѧ������ʵ��װ�ý���ʵ�飬ʵ���й۲쵽��Ϸ�ĩ���ձ�ɺ�ɫ���ʣ�ͬʱ����һ����ɫ���д̼�����ζ�����壬�����廯ѧʽΪ_______��������Ϊ��ͬѧ��ʵ��װ������װB���ʵ��Թ����ձ�֮�����һ��������װ�ã�����Ϊ�Ƿ��б�Ҫ_____�����б�Ҫ�����ԭ���ԸĽ�____________��

��15�֣���1��2Fe2����H2O2��4OH����2Fe(OH)3��2�֣�
��2�����ˡ�ϴ�ӡ����2�֣�ֻ���һ�����÷֣���2����1�֣���3��CuO��2�֣������ȶ���2�֣�
��Ӳ�ʲ������к�ɫ�����ɺ�ɫ���ձ��е��ܿڲ��ֲ������ݣ�����ɫ����ζ�������ɣ�2�֣�
��SO2��2�֣�����Ҫ��1�֣���ʵ������Ϊ��Ӧ����SO2��������NaOH��Һ��Ӧ����ʹ��ϵ��ѹǿ�����С��ɵ�����1�֣����ڵ���ĩ������һ���õ�©����1�֣���©����Ե�ո�û��Һ���£�1�֣�������ĩ������һ����ܣ��ڸ���ܵü��첿�ֲ���Һ���£����������Ҳ�÷֣�
��2�����ˡ�ϴ�ӡ����2�֣�ֻ���һ�����÷֣���2����1�֣���3��CuO��2�֣������ȶ���2�֣�
��Ӳ�ʲ������к�ɫ�����ɺ�ɫ���ձ��е��ܿڲ��ֲ������ݣ�����ɫ����ζ�������ɣ�2�֣�
��SO2��2�֣�����Ҫ��1�֣���ʵ������Ϊ��Ӧ����SO2��������NaOH��Һ��Ӧ����ʹ��ϵ��ѹǿ�����С��ɵ�����1�֣����ڵ���ĩ������һ���õ�©����1�֣���©����Ե�ո�û��Һ���£�1�֣�������ĩ������һ����ܣ��ڸ���ܵü��첿�ֲ���Һ���£����������Ҳ�÷֣�
�����������1���������Ӿ��л�ԭ�ԣ�˫��ˮ���������ԣ��ڼ��������£�������������������������������ȥ����Ӧ�����ӷ���ʽ��2Fe2����H2O2��4OH����2Fe(OH)3��
��2������������������������������������������������Ʊ�����������Ҫ�����ǣ�����2�ܽ�������������Һ�����˵õ������������壬Ȼ������Һ�н���������ϡ����������������Ȼ������Ũ������ȴ�ᾧ���ɣ�����ʵ����̵�������ǹ��ˡ�ϴ�ӡ����
��3��A�����������0.80g������ڼ��ȹ����й������0.98g��0.80g��0.18g������0.98g������ͭ��ˮ��������
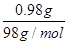 ��18g/mol��0.18g����˵��A����ǡ��������ͭ����ѧʽ��CuO������ͭ��������������������ͭ����˵���ڸ����£�������ͭ������ͭ���ȶ���
��18g/mol��0.18g����˵��A����ǡ��������ͭ����ѧʽ��CuO������ͭ��������������������ͭ����˵���ڸ����£�������ͭ������ͭ���ȶ���������ͭ�ɽ�NH3����ΪN2��ͬʱ����ͭ����ԭΪ����Cu������֧�ּ�ͬѧ���۵�������Ӳ�ʲ������к�ɫ�����ɺ�ɫ���ձ��е��ܿڲ��ֲ������ݣ�����ɫ����ζ�������ɡ�
�����ڸ���������ɫ���д̼�����ζ�����壬���Ը������ʵ����Ԫ�ؿ�֪����������SO2������ʵ������Ϊ��Ӧ����SO2��������NaOH��Һ��Ӧ����ʹ��ϵ��ѹǿ�����С��ɵ����������ڵ���ĩ������һ���õ�©����©����Ե�ո�û��Һ�����Է�ֹ�������������б�Ҫ�ġ�2�����ʡ�����װ�õ�ѡ���

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ
 ��ʽ����
��ʽ����