��Ŀ����
(��15��) �������ͼ����
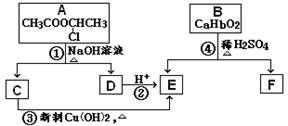
��֪��һ��̼ԭ�������������ǻ�ʱ����������ת����
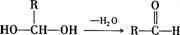
��1����Ӧ���������л���Ӧ������_______________��Ӧ��
��2����Ӧ�۵Ļ�ѧ����ʽ______________________________________________________��
��3����֪B����Է�������Ϊ162������ȫȼ�յIJ�����n(CO2)��n (H2O) = 2��1����B�ķ���ʽΪ ��
��4��F�Ǹ߷��ӹ���������е���Ҫԭ�ϡ�F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ���۱����ϵ�һ�ȴ���ֻ�����֡�F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������G��F��ͬ���칹�壬���ڷ����廯����ܷ���������Ӧ��G�Ľṹ�� �֡�
��6��������H��B��ͬ���칹�壬H�����к��еIJ��ֽṹΪ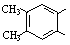 ������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾���(CaHbO2)n.��H�ж��ֽṹ��д������һ�ֵĽṹ��ʽ
��
������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾���(CaHbO2)n.��H�ж��ֽṹ��д������һ�ֵĽṹ��ʽ
��
��1��ȡ������ˮ�⣩��1�֣�
��2��CH3CHO + 2Cu(OH)2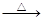 CH3COOH
+ Cu2O��+ 2H2O(3��)
CH3COOH
+ Cu2O��+ 2H2O(3��)
��3��C10H10O2(2��)
��4��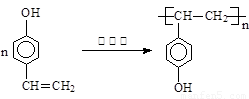 (3��)
(3��)
��5��4��3�֣�
��6��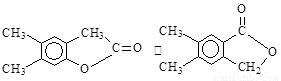 ��3�֣�
��3�֣�
��������

��1����ÿ��1�֣���1����3�֣������2�֣���15�֣�
ijУ��ѧ����С�������������ṩ������װ�ú�ҩƷ��ȡNaHCO3��Һ���������ʵ�顣ʵ�����ṩ����ҩƷ����2%NaOH��Һ��ϡHCl��ϡH2SO4�ܱ���KHCO3��Һ��ŨH2SO4��CaCO3�����K2CO3��ĩ�ിˮ���ṩ��������װ�ã�
�������ĿҪ��ش��������⣺
��l���밴�±�Ҫ����дѡ���װ�ú�ҩƷ
| ���� ���� | CO2����װ�ã�X�� �濪���ã������ͣ | ����ϴ��װ�ã�Y�� | �Ʊ���Ʒװ�ã�Z�� |
| ѡ���װ�ã�����ţ� | | | C |
| ѡ���ҩƷ������ţ� | | | �� |
 ����װ��(X)�������ԣ���д����Ҫ�������̣�
����װ��(X)�������ԣ���д����Ҫ�������̣�_____________________________________________________________________________
__________________________________________________________________________��
(3)��װ�ð�X��Y��Z˳�����Ӳ���������Ժ�����ҩƷʵ��ʱ��Xװ���з�����ѧ��Ӧ�����ӷ���ʽΪ________________��Yװ���г�ȥ������Ϊ_____________��
(4)�����£���Zװ�õ�NaOH��Һ��ͨ�����
 ���壬��ԭ����_________________��ͨ�����
���壬��ԭ����_________________��ͨ����� ��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________ ��
��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________ �� ��1����ÿ��1�֣���1����3�֣������2�֣���15�֣�
ijУ��ѧ����С�������������ṩ������װ�ú�ҩƷ��ȡNaHCO3��Һ���������ʵ�顣ʵ�����ṩ����ҩƷ����2%NaOH��Һ��ϡHCl��ϡH2SO4�ܱ���KHCO3��Һ��ŨH2SO4��CaCO3�����K2CO3��ĩ�ിˮ���ṩ��������װ�ã�

�������ĿҪ��ش��������⣺
��l���밴�±�Ҫ����дѡ���װ�ú�ҩƷ
|
���� ���� |
CO2����װ�ã�X�� �濪���ã������ͣ |
����ϴ��װ�ã�Y�� |
�Ʊ���Ʒװ�ã�Z�� |
|
ѡ���װ�ã�����ţ� |
|
|
C |
|
ѡ���ҩƷ������ţ� |
|
|
�� |
(2)��μ�����ѡ��� ����װ��(X)�������ԣ���д����Ҫ�������̣�
����װ��(X)�������ԣ���д����Ҫ�������̣�
_____________________________________________________________________________
__________________________________________________________________________��
(3)��װ�ð�X��Y��Z˳�����Ӳ���������Ժ�����ҩƷʵ��ʱ��Xװ���з�����ѧ��Ӧ�����ӷ���ʽΪ________________��Yװ���г�ȥ������Ϊ_____________��
(4)�����£���Zװ�õ�NaOH��Һ��ͨ����� ���壬��ԭ����_________________��ͨ�����
���壬��ԭ����_________________��ͨ����� ��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________
��
��Zװ���ڵ���Һ������Ũ�ȴ�С˳��Ϊ��_______________________
��

