��Ŀ����
����Ŀ������Ӧ�ù㷺�Ľ�����������������Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3��������Ϊԭ���Ʊ�����һ�ֹ�������������
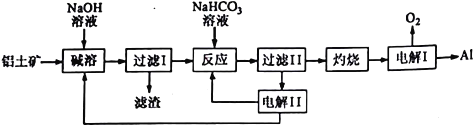
ע��SiO2����������ʱת��Ϊ�������Ƴ�����
��1��A1��ԭ�ӽṹʾ��ͼΪ_______________��A1��NaOH��Һ��Ӧ�����ӷ���ʽΪ______________________________________��
��2�� ��������ʱ����ƫ�����Ƶ����ӷ���ʽΪ_______________________________��
��3������ ������������Һ�м���NaHCO3��Һ����Һ��pH_________���������������� ������������С������
��4�� ��������ǵ������Al2O3������������������ʯī�����ģ�ԭ����__________________��
��5���������ԭ����ͼ��ʾ��
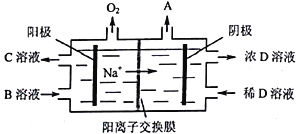
����д��A��B��C��D���ʵĻ�ѧʽ��A_______��B_______��C_______��D_______��
�������ĵ缫��ӦʽΪ______________________________��
��6��������1000��ʱ����N2��Ӧ�Ʊ�AlN������������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ�������Ҫԭ����________________________________��
���𰸡� 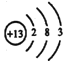 2Al+2H2O+2OH-=2AlO2-+3H2�� Al2O3+2OH-= 2AlO2-+H2O ��С ʯī�缫�������ϲ������������� H2 Na2CO3 NaHCO3 NaOH �������10���������10��4CO32-+2H2O4e-=4HCO3-+O2�� �Ȼ�立ֽ�������Ȼ����ܹ��ƻ����������������Ĥ
2Al+2H2O+2OH-=2AlO2-+3H2�� Al2O3+2OH-= 2AlO2-+H2O ��С ʯī�缫�������ϲ������������� H2 Na2CO3 NaHCO3 NaOH �������10���������10��4CO32-+2H2O4e-=4HCO3-+O2�� �Ȼ�立ֽ�������Ȼ����ܹ��ƻ����������������Ĥ
����������1��A1��ԭ�ӽṹʾ��ͼΪ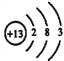 ��A1��NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2H2O+2OH-=2AlO2-+3H2 ������2�� ��������ʱ��������Ӧ����ƫ�����ƣ����ӷ���ʽΪ��Al2O3+2OH-=2 AlO2-+H2O ����3�����ˢ�������Һ�к����������ơ�ƫ�����ƣ�������NaHCO3��Һ���������·�Ӧ��OH- +HCO3-=CO32- +H2O��AlO2-+H2O+ HCO3-= CO32- +Al(OH)3������Һ��pH��С����4�� ���������ǵ������Al2O3������������������ʯī�����ģ�����Ϊ���ɵ���������������̼��Ӧ����5���ɵ���ԭ��ͼ�����ʻ��ϼ۱仯������̼��������ӵ���Ϣ������֪����A��H2��B�� Na2CO3��C��NaHCO3��D��NaOH���������ĵ缫��ӦʽΪ��4CO32-+2H2O4e-=4HCO3-+O2������6��������1000��ʱ����N2��Ӧ�Ʊ�AlN�ǻ������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ���Ӧ�����Ȼ�������ã�������1000�滷���У��Ȼ�立ֽ�Ϊ�Ȼ����ܹ��ƻ����������������Ĥ���ٽ����ϸ����С�
��A1��NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2H2O+2OH-=2AlO2-+3H2 ������2�� ��������ʱ��������Ӧ����ƫ�����ƣ����ӷ���ʽΪ��Al2O3+2OH-=2 AlO2-+H2O ����3�����ˢ�������Һ�к����������ơ�ƫ�����ƣ�������NaHCO3��Һ���������·�Ӧ��OH- +HCO3-=CO32- +H2O��AlO2-+H2O+ HCO3-= CO32- +Al(OH)3������Һ��pH��С����4�� ���������ǵ������Al2O3������������������ʯī�����ģ�����Ϊ���ɵ���������������̼��Ӧ����5���ɵ���ԭ��ͼ�����ʻ��ϼ۱仯������̼��������ӵ���Ϣ������֪����A��H2��B�� Na2CO3��C��NaHCO3��D��NaOH���������ĵ缫��ӦʽΪ��4CO32-+2H2O4e-=4HCO3-+O2������6��������1000��ʱ����N2��Ӧ�Ʊ�AlN�ǻ������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ���Ӧ�����Ȼ�������ã�������1000�滷���У��Ȼ�立ֽ�Ϊ�Ȼ����ܹ��ƻ����������������Ĥ���ٽ����ϸ����С�