��Ŀ����
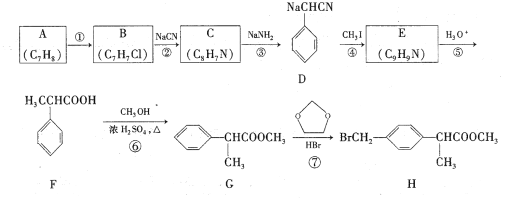
����Ŀ����ҵ�������β���к���һ����SO2������β�����ֶγ��˰����շ���������¼��ַ�����
��һ����ҵʵ�����չ����У��ڢ�����Ũ��������Һ����Ϊ���е�SO2��Ȼ����������Һ�м�����ʯ�ң���ַ�Ӧ�����ɲ��������پ��������Ƶò�ƷA��
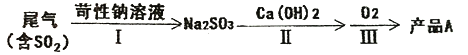
��1����ƷA��_______���ѧʽ����
��2�������������һ���е�Ũ��������Һ����ͬ�¶��±���Ca(OH)2��Һֱ���Ƶò�ƷCaSO3������Ϊ�Ƿ���У�_______������ԡ����������ԡ���ȷ������ԭ����_______��
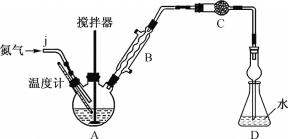
�������Ʊ�MnSO4H2O��SO2��ԭMnO2���Ʊ�MnSO4H2O���������£�

��֪25��ʱ�������ʵ��ܶȻ��������±�:
���� | Mn(OH)2 | Fe(OH)3 | Cu(OH)2 | MnS | FeS | CuS |
�ܶȻ� | 2.1��10-13 | 4.0�� 10-38 | 2.2��10-23 | 1.0��10-11 | 6.3��10-18 | 6.3��10-38 |
��3����ԭʱ����β����SO2����Ϊ4.48%,��ȥ�����lm3β��������Ҫ������������Ϊ55%��MnO2 ���_______g��
��4������ʱ����̼��ƣ������ķ�Ӧ�����ӷ�Ӧ����ʽΪ_____________________��
��5���Լ�A�����______________��д��ѧʽ����
��6������I������Ϊ_________________��MnSO4H2O��1150��������ֽ⣬������Mn3O4�������ˮ���ڸ������������̾���ֽⷴӦ�Ļ�ѧ����ʽ��________________��
���𰸡� CaSO4 ������ Ca(OH)2��ˮ���ܽ�Ƚ�С��c(OH-)̫�ͣ�����Ч�ʲ��� 200 2Fe3++3H2O+3CaCO3=2 Fe(OH)3��+3CO2��+3Ca2+����2Fe3++3H2O![]() Fe(OH)3+3H+ 2H++3CaCO3=Ca2++CO2��+H2O�� MnS ����Ũ������ȴ�ᾧ 3MnSO4��H2O
Fe(OH)3+3H+ 2H++3CaCO3=Ca2++CO2��+H2O�� MnS ����Ũ������ȴ�ᾧ 3MnSO4��H2O![]() Mn3O4+SO2��+2SO3��+3H2O
Mn3O4+SO2��+2SO3��+3H2O
�������������������һ����1������Ũ��������Һ����Ϊ���е�SO2��Ȼ����������Һ�м�����ʯ�ң���ַ�Ӧ�������������ƣ�������پ��������Ƶ�����ƣ�
��2��Ũ��������Һ����ͬ�¶��±���Ca��OH��2��Һֱ���Ƶò�ƷCaSO3ʱ�����������ܽ�Ƚ�С�����ն��������Ч�ʲ��ߣ�
�����������Ʊ�MnSO4H2O��ԭ����������SO2��ԭMnO2���Ʊ�MnSO4H2O��
��3�����ݻ�ѧ����ʽ�������㣻
��4�������̼�����Ϊ�˺������ӷ�Ӧʹ��ҺPH���ߵ�6.5,ʹ����������ˮ������������������5��һ�ֳ�����ת��Ϊ�����ܵij��������ó���ת�����ؽ������ӣ�
��6���������̷���������I�ǶԵõ�����������Һ�ᾧ����������Ϊ�����ܼ�Ũ����ȴ�ᾧ�� MnSO4H2O��1150��������ֽ⣬������Mn3O4�������ˮ�����ݻ��ϼ۵�������ͬ��ԭ���غ���д��ѧ����ʽ��
��������һ����1�����̷����ķ�Ӧ�Ƕ���������������Ʒ�Ӧ�����������ƣ��������ƺ�����������Ӧ����������ƣ�������Ʊ���������Ϊ����ƣ���ѧʽΪ��CaSO4��
��2������һ���е�Ũ��������Һ����ͬ�¶��±���Ca��OH��2��Һֱ���Ƶò�ƷCaSO3���������������ܽ��С�Զ����������ղ���ȫ�����Բ����ԣ�
��������3��lm3β������SO2��1000L��4.48%��22.4L/mol=2mol����Ҫ������������Ϊ55%��MnO2 ���xg
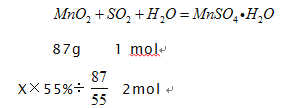
X=200g
��4�����������ķ�Ӧ��������������PH=6.5ʱȫ��������ȥ������������ˮ���������������������ӣ�����̼��ƻ�������ӷ�Ӧ��ʹPH���ߵ�6.5�����Է�Ӧ�����ӷ�Ӧ����ʽΪ��2Fe3++3H2O+3CaCO3=2Fe��OH��3��+3CO2��+3Ca2+��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ��������ʵ���������ƥ�����
ʵ����� | ʵ������ | |
A | ��ʢ�и������������Һ���Թ���ͨ����������ϩ���� | ��Һ����ɫ����ȥ�����ú���Һ�ֲ� |
B | ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в���Ũ�̲��к�ɫ�������� |
C | ��ʢ�б��������������Һ���Թ��еμ�ϡ���� | �д̼�����ζ�����������Һ����� |
D | ��ʢ��FeCl3��Һ���Թ��мӹ������ۣ�������1��KSCN��Һ | ��ɫ����ʧ����KSCN����Һ��ɫ���� |
A. AB. BC. CD. D