��Ŀ����
I�����Ȼ�����S2Cl2���ǹ㷺������ҵ��������������һ�ֳȻ�ɫ�ж����Һ�壬���ķ��ӽṹ��H2O2���ƣ���ˮ��ˮ�⣬������ʹƷ����ɫ�����壮S2Cl2���ɸ�������ͨ�����ڵ������Ƶã�
��1��д�����Ȼ�����S2Cl2���ĵ���ʽ______�����Ȼ�����S2Cl2�������ľ�������______��
��2��д�����Ȼ�����S2Cl2����ˮ��Ӧ�Ļ�ѧ����ʽ______��
II��пԪ�����˵����������й�ϵ����ZnSO4?7H2O��ZnO�Ⱦ�����ҩ���ֵ��
��1����ҵ���Ʊ�ZnSO4?7H2O�ķ����ܶ࣬���ɴ�ZnO����Fe2+��Mn2+��Cd2+��Ni2+��ͨ������������ɣ�
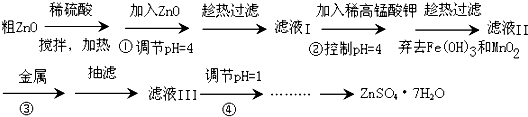
����д������ϡ���������Һʱ�����ӷ���ʽ��______��______��
�����̢��еĽ���������______
A��Na������������B��Al������������C��Zn������������ D��Cu
�����̢��е�pH=1Ŀ��Ϊ______��
��2������п���Ʊ���ȡ���Ƶ�����п��Һ��������̼������Һ���ӱ߽���ʹ������ȫ�ݳ�����ʹpHԼΪ6.8���ң������������������ȥ�ϲ���Һ������ˮϴ�ӵ���SO42-Ϊֹ�����˸����ù���ZnCO3?2Zn��OH��2?2H2Oת������A�У���������ȫ�ֽ⣬��ȴ�ù���ZnO��
������A����Ϊ______��
��д������п��Һ����������̼������Һʱ�Ļ�ѧ����ʽ______��
�����֤������A����������ȫ�ֽ⣿______��
�⣺��1�����ӽṹ��H2O2���ƣ�S2Cl2��չ����ҳ�ͽṹ��Cl-Sλ��������ҳ���ڣ�S2Cl2������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Clԭ����Sԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ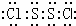 ��
��
���ӽṹ��H2O2���ƣ�������ΪҺ�壬���ڷ��Ӿ��壻
�ʴ�Ϊ��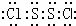 �����Ӿ��壻
�����Ӿ��壻
��2��S2Cl2��ˮ��ˮ�⣬��������ʹƷ����Һ��ɫ�����壬������Ϊ���������ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO2����һ���ֽ��͵�0�ۣ�����S����ͬʱ����HCl����Ӧ����ʽΪ��2S2Cl2+2H2O=3S��+SO2��+4HCl��
�ʴ�Ϊ��2S2Cl2+2H2O=3S��+SO2��+4HCl��
��1�����ɹ������̿�֪�����������������������������������ɣ�Ӧ�Ǹ���������������������������������������̣���������������������ɶ������̣����ݿ��Ƶ�PHֵ��ԭ���غ㡢����غ��֪������ˮ�μӷ�Ӧ��ͬʱ�����������ӣ���Ӧ�����ӷ���ʽΪ��MnO4-+3Fe2++7H2O=3Fe��OH��3��+MnO2��+5H+��2MnO4-+3Mn2++2H2O=5MnO2��+4H+��
�ʴ�Ϊ��MnO4-+3Fe2++7H2O=3Fe��OH��3��+MnO2��+5H+��2MnO4-+3Mn2++2H2O=5MnO2��+4H+��
�����̢��м��������Ŀ�ģ�Ӧ�dz�ȥ��Һ�н������ӣ��ֲ����������ʣ�Ӧ��ѡ��п��
�ʴ�Ϊ��C��
��п����ˮ�⣬���̢��е�pH=1Ŀ��������Zn2+��ˮ�⣬�ʴ�Ϊ������Zn2+��ˮ�⣻
��2�������չ��壬Ӧ���������ʴ�Ϊ��������
�ڷ�Ӧ����ZnCO3?2Zn��OH��2?2H2O�����ɵ�����Ϊ������̼������Ԫ���غ��֪���������������ɣ���Ӧ����ʽΪ��3ZnSO4+3Na2CO3+4H2O=ZnCO3?2Zn��OH��2?2H2O��+3Na2SO4+2CO2����
�ʴ�Ϊ��3ZnSO4+3Na2CO3+4H2O=ZnCO3?2Zn��OH��2?2H2O��+3Na2SO4+2CO2����
�ۼ������պ�������Ƿ���̼������ɣ�ʵ�����Ϊ��ȡ�������յĹ��壬����ϡ���ᣬ�۲��Ƿ������ݲ������������ݲ�����˵������ȫ�ֽ⣬
�ʴ�Ϊ��ȡ�������յĹ��壬����ϡ���ᣬ�۲��Ƿ������ݲ������������ݲ�����˵������ȫ�ֽ⣮
��������1�����ӽṹ��H2O2���ƣ�S2Cl2��չ����ҳ�ͽṹ��Cl-Sλ��������ҳ���ڣ�S2Cl2������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Clԭ����Sԭ��֮���γ�1�Թ��õ��Ӷԣ�
���ӽṹ��H2O2���ƣ����ڷ��Ӿ��壻
��2��S2Cl2��ˮ��ˮ�⣬��������ʹƷ����Һ��ɫ�����壬������Ϊ���������ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO2����һ���ֽ��͵�0�ۣ�����S����ͬʱ����HCl��
��1�����ɹ������̿�֪�����������������������������������ɣ�Ӧ�Ǹ���������������������������������������̣���������������������ɶ������̣����ݿ��Ƶ�PHֵ��ԭ���غ㡢����غ��֪������ˮ�μӷ�Ӧ��ͬʱ�����������ӣ�
�����̢��м��������Ŀ�ģ�Ӧ�dz�ȥ��Һ�н������ӣ��ֲ����������ʣ�Ӧ��ѡ��п��
��п����ˮ�⣬���̢��е�pH=1Ŀ��������Zn2+��ˮ�⣻
��2�������չ��壬Ӧ��������
�ڷ�Ӧ����ZnCO3?2Zn��OH��2?2H2O�����ɵ�����Ϊ������̼������Ԫ���غ��֪���������������ɣ�
�ۼ������պ�������Ƿ���̼������ɣ��������ᷴӦ���������жϣ�
���������⿼�黯ѧ�����������̡�����Ϣ����ȡ���õȣ���Ŀ�ѶȽϴ����ҵ�����ǽ���Ĺؼ���ע��������ʵ�������������ԭ������Ҫѧ��������ʵ�Ļ���������֪ʶ���ӽ�������������
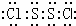 ��
�����ӽṹ��H2O2���ƣ�������ΪҺ�壬���ڷ��Ӿ��壻
�ʴ�Ϊ��
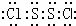 �����Ӿ��壻
�����Ӿ��壻��2��S2Cl2��ˮ��ˮ�⣬��������ʹƷ����Һ��ɫ�����壬������Ϊ���������ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO2����һ���ֽ��͵�0�ۣ�����S����ͬʱ����HCl����Ӧ����ʽΪ��2S2Cl2+2H2O=3S��+SO2��+4HCl��
�ʴ�Ϊ��2S2Cl2+2H2O=3S��+SO2��+4HCl��
��1�����ɹ������̿�֪�����������������������������������ɣ�Ӧ�Ǹ���������������������������������������̣���������������������ɶ������̣����ݿ��Ƶ�PHֵ��ԭ���غ㡢����غ��֪������ˮ�μӷ�Ӧ��ͬʱ�����������ӣ���Ӧ�����ӷ���ʽΪ��MnO4-+3Fe2++7H2O=3Fe��OH��3��+MnO2��+5H+��2MnO4-+3Mn2++2H2O=5MnO2��+4H+��
�ʴ�Ϊ��MnO4-+3Fe2++7H2O=3Fe��OH��3��+MnO2��+5H+��2MnO4-+3Mn2++2H2O=5MnO2��+4H+��
�����̢��м��������Ŀ�ģ�Ӧ�dz�ȥ��Һ�н������ӣ��ֲ����������ʣ�Ӧ��ѡ��п��
�ʴ�Ϊ��C��
��п����ˮ�⣬���̢��е�pH=1Ŀ��������Zn2+��ˮ�⣬�ʴ�Ϊ������Zn2+��ˮ�⣻
��2�������չ��壬Ӧ���������ʴ�Ϊ��������
�ڷ�Ӧ����ZnCO3?2Zn��OH��2?2H2O�����ɵ�����Ϊ������̼������Ԫ���غ��֪���������������ɣ���Ӧ����ʽΪ��3ZnSO4+3Na2CO3+4H2O=ZnCO3?2Zn��OH��2?2H2O��+3Na2SO4+2CO2����
�ʴ�Ϊ��3ZnSO4+3Na2CO3+4H2O=ZnCO3?2Zn��OH��2?2H2O��+3Na2SO4+2CO2����
�ۼ������պ�������Ƿ���̼������ɣ�ʵ�����Ϊ��ȡ�������յĹ��壬����ϡ���ᣬ�۲��Ƿ������ݲ������������ݲ�����˵������ȫ�ֽ⣬
�ʴ�Ϊ��ȡ�������յĹ��壬����ϡ���ᣬ�۲��Ƿ������ݲ������������ݲ�����˵������ȫ�ֽ⣮
��������1�����ӽṹ��H2O2���ƣ�S2Cl2��չ����ҳ�ͽṹ��Cl-Sλ��������ҳ���ڣ�S2Cl2������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Clԭ����Sԭ��֮���γ�1�Թ��õ��Ӷԣ�
���ӽṹ��H2O2���ƣ����ڷ��Ӿ��壻
��2��S2Cl2��ˮ��ˮ�⣬��������ʹƷ����Һ��ɫ�����壬������Ϊ���������ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO2����һ���ֽ��͵�0�ۣ�����S����ͬʱ����HCl��
��1�����ɹ������̿�֪�����������������������������������ɣ�Ӧ�Ǹ���������������������������������������̣���������������������ɶ������̣����ݿ��Ƶ�PHֵ��ԭ���غ㡢����غ��֪������ˮ�μӷ�Ӧ��ͬʱ�����������ӣ�
�����̢��м��������Ŀ�ģ�Ӧ�dz�ȥ��Һ�н������ӣ��ֲ����������ʣ�Ӧ��ѡ��п��
��п����ˮ�⣬���̢��е�pH=1Ŀ��������Zn2+��ˮ�⣻
��2�������չ��壬Ӧ��������
�ڷ�Ӧ����ZnCO3?2Zn��OH��2?2H2O�����ɵ�����Ϊ������̼������Ԫ���غ��֪���������������ɣ�
�ۼ������պ�������Ƿ���̼������ɣ��������ᷴӦ���������жϣ�
���������⿼�黯ѧ�����������̡�����Ϣ����ȡ���õȣ���Ŀ�ѶȽϴ����ҵ�����ǽ���Ĺؼ���ע��������ʵ�������������ԭ������Ҫѧ��������ʵ�Ļ���������֪ʶ���ӽ�������������

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д�
�����Ŀ
����ʵ����������Ӧ���۾���ȷ����
| ѡ�� | ���� | ���� | ���� |
| A | ��Ũ����ε����DZ��� | ���������� | Ũ��������ˮ�Ժ�ǿ������ |
| B | �����½�AlƬ����Ũ������ | �����Ա仯 | Al��Ũ�����Ӧ |
| C | ��һС��Na������ˮ�Ҵ��� | �������� | Na���û������ǻ��е��� |
| D | ��ˮ����ͨ�����ȵ����� | ��ĩ��� | ����ˮ�ڸ����·�����Ӧ |
- A.A
- B.B
- C.C
- D.D
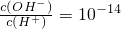 ����Һ�У�Al3+��NH4+��Cl-��CO32-
����Һ�У�Al3+��NH4+��Cl-��CO32-