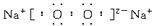��Ŀ����
��10�֣���֪�����и������ʶ���1~18��Ԫ����ɣ�����֮��Ĺ�ϵ����ͼ��ʾ��
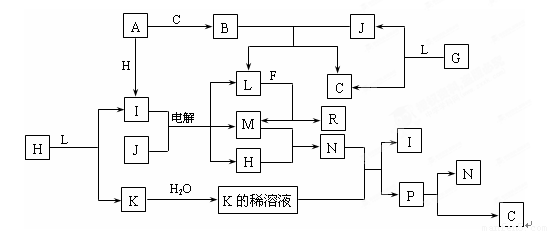
�����£�A��FΪ�������ʣ�J��Һ�壬F������L��Һ��Ӧ��������N��Һ��Ӧ��
C��H��MΪ���嵥�ʣ�����H�ʻ���ɫ��A��B��I��K��L��R����ɫ��Ӧ���ʻ�ɫ��
R��ˮ��Һ�еμ�����ʱ���տ�ʼ�а�ɫ�������������������ܽ⡣
��֪G��H2O2��H2O2�ڼ��������·ֽ����ʻ�ӿ졣��ش�
��1��BΪ����ɫ���壬��д��B��J��Ӧ�����ӷ���ʽ
��2��P���ȶ��ֽ��N��C���÷�Ӧ�Ļ�ѧ����ʽΪ
��3����д��������ⷴӦ�Ļ�ѧ����ʽ
��4��R���������ᷴӦ�����ӷ���ʽΪ
��5��F�dz�����Ұ�⺸���������ԭ�ϣ���д�����������������Ļ�ѧ��Ӧ����ʽ��
��1��2Na2O2 + 2H2O == 4Na+ + 4OH�� + O2�� (2) 2HClO == 2HCl + O2��
��3��2NaCl + 2H2O == 2NaOH + H2��+ Cl2��
 ��4��AlO2�� +
4H+ == Al3+ + 2H2O
��4��AlO2�� +
4H+ == Al3+ + 2H2O
(5) 2Al + Fe2O3 === Al2O3 + 2Fe �� 8Al + 3Fe3O4 === 4Al2O3 + 9Fe
����������

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�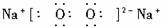

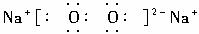 ?
?