��Ŀ����
�����й����ӷ�Ӧ�����ӷ���ʽ�������У���ȷ����
| A����ʹpH��ֽ�����ɫ����Һ�У�Fe3����Cl����Ba2����Br���ܴ������� |
B�����Ե缫����Ȼ�����Һ�� |
C��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ4Mg��6H����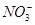 =4Mg2���� =4Mg2����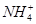 ��3H2O ��3H2O |
| D����10 mL 0.1 mol��L��1 KAl(SO4)2��Һ��10 mL 0.2 mol��L��1Ba(OH)2��Һ��ϣ��õ��ij�����Al(OH)3��BaSO4�����ʵ���֮��Ϊ1��2 |
A
��ʹpH��ֽ�����ɫ����Һ�����ԣ�A�����漰�����ӿ��Դ������棻�ں�Al3������Һ�в���������OH����B�����C���ɲ��غ㣻D����KAl(SO4)2��Ba(OH)2�����ʵ�����Ϊ1��2�����ǵ������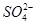 ��Ba2�������ʵ�����Ϊ1��1��ǡ����ȫ����������BaSO4����Al3����OH�������ʵ�����Ϊ1��4��ǡ����ȫ��Ӧ����
��Ba2�������ʵ�����Ϊ1��1��ǡ����ȫ����������BaSO4����Al3����OH�������ʵ�����Ϊ1��4��ǡ����ȫ��Ӧ����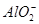 �������ó����в���Al(OH)3��
�������ó����в���Al(OH)3��
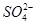 ��Ba2�������ʵ�����Ϊ1��1��ǡ����ȫ����������BaSO4����Al3����OH�������ʵ�����Ϊ1��4��ǡ����ȫ��Ӧ����
��Ba2�������ʵ�����Ϊ1��1��ǡ����ȫ����������BaSO4����Al3����OH�������ʵ�����Ϊ1��4��ǡ����ȫ��Ӧ����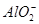 �������ó����в���Al(OH)3��
�������ó����в���Al(OH)3��
��ϰ��ϵ�д�
�����Ŀ
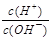 ��1��10��12����Һ�� K����AlO2����CO32����Na���������ӿ��Դ�������
��1��10��12����Һ�� K����AlO2����CO32����Na���������ӿ��Դ�������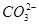 ��
�� ��
�� ��Cl��
��Cl�� ��Na��
��Na��
 Sn(OH)2
Sn(OH)2 ��CH3CH2OH��
��CH3CH2OH��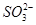
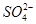
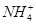 ��
��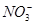 ��Cl��
��Cl��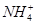 ��Cl����
��Cl����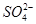 ��
��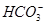 ��
��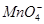 �еļ��֡�Ϊȷ����ɷ֣�������ʵ�飺��ȡ������Һ������������Na2O2���壬������ɫ��ζ������Ͱ�ɫ�������ټ���������NaOH��Һ���ɫ���������ܽ⣻����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ���������������ƶ���ȷ����
�еļ��֡�Ϊȷ����ɷ֣�������ʵ�飺��ȡ������Һ������������Na2O2���壬������ɫ��ζ������Ͱ�ɫ�������ټ���������NaOH��Һ���ɫ���������ܽ⣻����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ���������������ƶ���ȷ����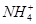 )/c(NH3��H2O)��ֵ����
)/c(NH3��H2O)��ֵ����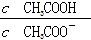 ��ֵ��С
��ֵ��С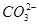 ˮ��̶ȼ�С����Һ��pH��С
ˮ��̶ȼ�С����Һ��pH��С