��Ŀ����
����ƽ�����������ϸ���һ����������ձ����ټ�������ʵ���Ũ�ȡ�����������ᣬ������ƽƽ�⣬Ȼ��һ���ձ��м���a g���ۣ���һ���ձ��м���b gþ�ۣ���ַ�Ӧ����ƽ�Ա���ƽ�⣬��ÿ���ձ��ж�����m mol H2SO4���ô���ʽ��ʾ���и��������a��b�Ĺ�ϵʽ��
(1)��a>56m��b>24mʱ_________��
(2)��a<56m��b<24mʱ_________��
(3)��a<56m��b>24mʱ_________��
(1)��a>56m��b>24mʱ_________��
(2)��a<56m��b<24mʱ_________��
(3)��a<56m��b>24mʱ_________��
(1)a="b " (2)81a="77b " (3) =b-2m
=b-2m
 =b-2m
=b-2m(1)���ձ��У�H2SO4�����㣬�������H2��2m g����a-2m=b-2m?a=b��
(2)���ձ��У�H2SO4����������Fe��H2SO4��Ӧ����H2�� ��2 g��Mg��H2SO4��Ӧ����H2��
��2 g��Mg��H2SO4��Ӧ����H2��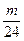 ��2 g������a-
��2 g������a- ��2=b-
��2=b-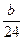 ��2.81a=77b��
��2.81a=77b��
(3)��Fe���ձ���H2SO4��������þ���ձ���H2SO4���㣬��Fe��H2SO4��Ӧ�ų�H2�� ��2 g��þ��H2SO4��Ӧ����H2��2m g����a-
��2 g��þ��H2SO4��Ӧ����H2��2m g����a- ��2=b-2m��
��2=b-2m��
(2)���ձ��У�H2SO4����������Fe��H2SO4��Ӧ����H2��
 ��2 g��Mg��H2SO4��Ӧ����H2��
��2 g��Mg��H2SO4��Ӧ����H2��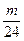 ��2 g������a-
��2 g������a- ��2=b-
��2=b-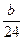 ��2.81a=77b��
��2.81a=77b��(3)��Fe���ձ���H2SO4��������þ���ձ���H2SO4���㣬��Fe��H2SO4��Ӧ�ų�H2��
 ��2 g��þ��H2SO4��Ӧ����H2��2m g����a-
��2 g��þ��H2SO4��Ӧ����H2��2m g����a- ��2=b-2m��
��2=b-2m��
��ϰ��ϵ�д�
�����Ŀ
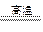 2Cu+CO2��
2Cu+CO2�� 2NaCl
2NaCl 2Fe+3CO2������3 mol e-ת�ƣ�����_______mol �����ɣ���_______��CO���Ӳμӷ�Ӧ��
2Fe+3CO2������3 mol e-ת�ƣ�����_______mol �����ɣ���_______��CO���Ӳμӷ�Ӧ�� ����ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� ��
����ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� �� (CO)>
(CO)>