��Ŀ����
���ἰ������ˮ�д����Ŷ���ƽ���ϵ��
��1����֪NaAˮ��Һ�ʼ��ԣ������½�0��10mol NaA��0��05 mol HCl����ˮ���õ�1L��Һ��
����֪�û����ҺΪ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ________________��
�����������Һ���ټ���0��03mol NaOH����Һ��n��A-����n��OH-����n��H+��=________mol��
��2��������̼��ˮ�г��˴���H2CO3��HCO3-�ĵ���ƽ���⣬������������ƽ�⣺����ѪҺ��pHͨ���ȶ���7��35��7��45֮�䣬���Ƕ������ع�ͬ���õĽ�������У�ѪҺ�д��ڵ�H2CO3-NaHCO3������ѪҺpH�����ȶ�����Ҫ���أ��ݴ˻ش�
�ٵ�c��H+������ʱ��ѪҺ������H+�����ӷ���ʽΪ___________________________��
�ڵ�c��OH-������ʱ��ѪҺ��pHҲ�ܱ��ֻ����ȶ����Խ�ϵ��뷽��ʽ��Ҫ˵����
___________________________________
��1����֪NaAˮ��Һ�ʼ��ԣ������½�0��10mol NaA��0��05 mol HCl����ˮ���õ�1L��Һ��
����֪�û����ҺΪ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ________________��
�����������Һ���ټ���0��03mol NaOH����Һ��n��A-����n��OH-����n��H+��=________mol��
��2��������̼��ˮ�г��˴���H2CO3��HCO3-�ĵ���ƽ���⣬������������ƽ�⣺����ѪҺ��pHͨ���ȶ���7��35��7��45֮�䣬���Ƕ������ع�ͬ���õĽ�������У�ѪҺ�д��ڵ�H2CO3-NaHCO3������ѪҺpH�����ȶ�����Ҫ���أ��ݴ˻ش�
�ٵ�c��H+������ʱ��ѪҺ������H+�����ӷ���ʽΪ___________________________��
�ڵ�c��OH-������ʱ��ѪҺ��pHҲ�ܱ��ֻ����ȶ����Խ�ϵ��뷽��ʽ��Ҫ˵����
___________________________________
��1����c��Na+����c��A-����c��Cl-����c��H+����c��OH-������0��08
��2����HCO3-+H+==H2CO3����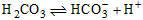 ��c��OH-������ʱ��H+��OH-��ϳ�ˮ��������
��c��OH-������ʱ��H+��OH-��ϳ�ˮ��������
OH-��ʹѪҺ��pH���ֻ����ȶ�
��2����HCO3-+H+==H2CO3����
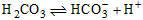 ��c��OH-������ʱ��H+��OH-��ϳ�ˮ��������
��c��OH-������ʱ��H+��OH-��ϳ�ˮ��������OH-��ʹѪҺ��pH���ֻ����ȶ�

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ