��Ŀ����
����֬�ް�ס0.2 g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵμ���ˮ���ɹ۲쵽��֬����ȼ��������
(1)������ʵ������ɵó������йع������Ƹ�ˮ��Ӧ�Ľ����ǣ�
��һ��____________________________________��
�ڶ���____________________________________��
����������ˮ��Ӧ�Ļ�ѧ����ʽ�ǣ�____________________________________��
��֬����Ҫ�ɷ���(C6H10O5)n��д����֬����ȫȼ�յĻ�ѧ��Ӧ����ʽ��________________��
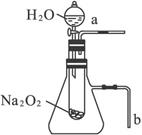
(2)ij�о���ѧϰС����������ͼ��ʾ��װ�ý���ʵ�飬����֤�������ۡ�
������֤��һ�����۵�ʵ�������____________________��������______________________��
������֤�ڶ������۵�ʵ�������______________________��������____________________��
(3)ʵ��(2)���Թ��м�ˮ��������ȫ�ܽ��Ҳ��ٲ������ݺ�ȡ���Թܣ����Թ��е���ʯ����Һ��������Һ����������ɫ��ȥ��Ϊ̽��������С��ͬѧ�Ӳ����й������е�֪���������Ƹ�ˮ��Ӧ�����ɹ������⣬�����������ǿ�����Ժ�Ư���ԡ������һ��ʵ�飬֤���������ƺ�����ˮ��ַ�Ӧ�����Һ���й���������ڡ�����������Һ������������Һ������������Һ���⻯����Һ����ɫ�����ȣ������ѡ���Լ���������֤(ֻҪ���г�ʵ�����õ��Լ����۲쵽��ʵ������)��
�Լ�_________________________������________________________��
(��)���������� �÷�ӦΪ���ȷ�Ӧ������һ�͵ڶ������ۿ��Եߵ����������(2)���е���֤����������Ҫ��Ӧ��
2Na2O2+2H2O====4NaOH+O2��
(C6H10O5)n+6nO2![]() 6nCO2+5nH2O
6nCO2+5nH2O
(2)�������ǵ�ľ�������ܿ�a ľ����ȼ ������b����ˮ�� ���ܿ������ݲ�����
(3)������������д�����һ��
| �� | �� | �� | �� | |
| �Լ� | ������Һ | ��ɫ���� | ����������Һ | �⻯����Һ |
| ���� | ��Һ����� | ������ɫ | ��Һ��dz��ɫ���ػ�ɫ | ��Һ����ɫ��Ϊ�غ�ɫ |
����:
���⿼��Կα�Ԫ�ؼ��仯����֪ʶ�����ա���������������չ��֪ʶ��Ǩ��������˼ά������ԡ�
�κ�����ȼ�ն���Ҫ������������֧��ȼ�յ����ʣ��ڴﵽ�����ʵ��Ż�㡣
(1)��֬����ȼ������˵��2Na2O2+2H2O====4NaOH+O2���������������ɣ��ڸ÷�ӦΪ���ȷ�Ӧ���ﵽ��֬���Ż�㡣
(2)һ��ͨ�ý������ǵ�ľ�������ܿڣ����ľ����ȼ��˵�����������ɣ���Ϊ�˷�Ӧ���ȣ����Ե�����ƿ���������ͣ����Ե���b����ˮ�У����ܿ������ݲ�����
(3)���ڹ�����������Ԫ��Ϊ-1�ۣ����Ծ���ǿ�����Ժ�Ư���ԡ�ʵ�ʿ��������������ԡ����Т٢ۢ����ù��������ǿ�����ԣ������ù��������Ư���ԡ�

|
����֬�ް�ס0.2 g��Na2O2��ĩ������ʯ�����ϣ�����֬���ϵμӼ���ˮ�����Թ۲쵽��֬����ȼ����������ʵ���������ó��Ľ�����ȷ���� | |
A�� |
Na2O2��H2O��Ӧ��һ�����ȷ�Ӧ |
B�� |
Na2O2��H2O��Ӧ������������ |
C�� |
Na2O2��H2O��Ӧ���������������� |
D�� |
Na2O2��H2O��Ӧ�����м����H2O2���� |
