��Ŀ����
��12�֣���ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ�

���ø�Ũ��������100 mL��1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У�
�ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ�����ȱ�ٵ�������
���� ��д�������ƣ���
(2)�����㣬����������ʵ���Ũ��Ϊ mol/L������100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL������һλС��������ȡŨ����ʱӦѡ�� ��ѡ���10mL����50mL ����100mL��������Ͳ��
��3���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����________������ţ���
A.��������Һ�壬ʹ��Һ����̶�������
B.С�ļ�������ƿ����������ʹ��Һ����̶�������
C.���������һ������Ũ����
D.��������
(ÿ��2��)(1) �� �� �� �� 100mL����ƿ ������
(2) 18.4 mol/L 5.4 �� ��3��D
����������1��������������Һ����� �� �� ���Dz���Ҫ�ġ���������ԭ����֪����ȱ��100mL����ƿ����������
��2������ ��֪�������Ũ����
��֪�������Ũ����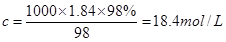 ����Ϊϡ�����У������Dz���ģ�������Ҫ����������
����Ϊϡ�����У������Dz���ģ�������Ҫ���������� �����ݹ�������ԭ���֪��Ӧ��ѡ��١�
�����ݹ�������ԭ���֪��Ӧ��ѡ��١�
��3���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ���˵��ʵ����֪ʧ�ܣ�ֻ����������ѡD��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д���ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ��ݴ�����˵����ȷ���ǣ� ��
| A�����Լ������ʵ���Ũ��Ϊ9.2 mol/L |
| B��������50mL��������ͭ��Ӧ���ɱ�״����10.3 L SO2 |
| C������250mL��4.6 mol/L��ϡ�����������62.5 mL |
| D����������������ˮ��Ϻ�������Һ����������С��49% |
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol?L-1��ϡ���ᣮ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ����ش��������⣺
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol?L-1��ϡ���ᣮ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ����ش��������⣺ ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ�����˵����ȷ���ǣ�������
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ�����˵����ȷ���ǣ�������

