��Ŀ����
(10��)W��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ(��ש��ɫ)��Z2O�ͺ�ɫ��ZO���������
(1)W����̬�⻯�ﻯѧʽΪ �����ķе��PH3�ķе� ��
����ߡ��͡���W����̬�⻯���ȶ��Ա�H2O(g)____ ____(�ǿ��������)��
(2)Y�Ļ�̬ԭ�Ӻ�������Ų�ʽ��________ ______��Y�ĵ�һ�����ܱ�X��______ __(���С��)��
(3)Y������������Ӧˮ�����Ũ��Һ��Z�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��
��
(4)��֪�������ݣ�
Fe(s)��O2(g)===FeO(s)����H����272.0 kJ��mol��1
2X(s)��O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1
X�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��___________ ___________________��
(1)W����̬�⻯�ﻯѧʽΪ �����ķе��PH3�ķе� ��
����ߡ��͡���W����̬�⻯���ȶ��Ա�H2O(g)____ ____(�ǿ��������)��
(2)Y�Ļ�̬ԭ�Ӻ�������Ų�ʽ��________ ______��Y�ĵ�һ�����ܱ�X��______ __(���С��)��
(3)Y������������Ӧˮ�����Ũ��Һ��Z�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��
��
(4)��֪�������ݣ�
Fe(s)��O2(g)===FeO(s)����H����272.0 kJ��mol��1
2X(s)��O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1
X�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��___________ ___________________��
(1)NH3������3�֣���
(2����3�֣���
(3)Cu��2H2SO4(Ũ)CuSO4��SO2����2H2O��2�֣���
(4)2Al(s)��3FeO(s)===Al2O3(s)��3Fe(s)����H����859.7 kJ��mol��1��2�֣���
(2����3�֣���
(3)Cu��2H2SO4(Ũ)CuSO4��SO2����2H2O��2�֣���
(4)2Al(s)��3FeO(s)===Al2O3(s)��3Fe(s)����H����859.7 kJ��mol��1��2�֣���
�����������Ҫ�����ǵ�������������W��N��Y��S��X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ����ݹ���ԭ����֪��ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p1,����Ԫ�ء�Z���γɺ�ɫ(��ש��ɫ)��Z2O�ͺ�ɫ��ZO��������������������ʿ�֪Ӧ����ͭ���������ZΪͭ��
��1�������д���������е�ߡ��ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ��
��2�����ݹ���ԭ����д��Y�Ļ�̬ԭ�Ӻ�������Ų�ʽ����1s22s22p63s23p4���ǽ�����Խǿ����һ������Խ��S�Ƿǽ�����ǿ�����ǽ��������Ե�һ������S�Ĵ�
��3��Ũ�����ڼ���ʱ������������ͭ������ʽΪCu��2H2SO4(Ũ)CuSO4��SO2����2H2O��
��4�������˹���ɵ�Ӧ�á��ɢ�Fe(s)��O2(g)===FeO(s)����H����272.0 kJ��mol��1�͢�2X(s)��O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1�ɼ��㣬���ڣ��١�3�ɵõ�2Al(s)��3FeO(s)===Al2O3(s)��3Fe(s)����H����859.7 kJ��mol��1��
��1�������д���������е�ߡ��ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ��
��2�����ݹ���ԭ����д��Y�Ļ�̬ԭ�Ӻ�������Ų�ʽ����1s22s22p63s23p4���ǽ�����Խǿ����һ������Խ��S�Ƿǽ�����ǿ�����ǽ��������Ե�һ������S�Ĵ�
��3��Ũ�����ڼ���ʱ������������ͭ������ʽΪCu��2H2SO4(Ũ)CuSO4��SO2����2H2O��
��4�������˹���ɵ�Ӧ�á��ɢ�Fe(s)��O2(g)===FeO(s)����H����272.0 kJ��mol��1�͢�2X(s)��O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1�ɼ��㣬���ڣ��١�3�ɵõ�2Al(s)��3FeO(s)===Al2O3(s)��3Fe(s)����H����859.7 kJ��mol��1��

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
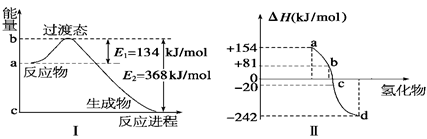
 2Fe(s)+3CO2(g) ��H="-a" kJ/mol
2Fe(s)+3CO2(g) ��H="-a" kJ/mol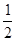 O2(g) �� H2O(g)����H1��a kJ��mol-1?
O2(g) �� H2O(g)����H1��a kJ��mol-1? 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a ��ֵ�� (д�� + ��)��
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a ��ֵ�� (д�� + ��)��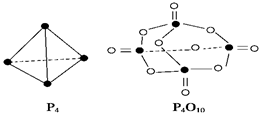
 O2��g����
O2��g���� P4O10��s�� ��H����738.5kJ��mol��1
P4O10��s�� ��H����738.5kJ��mol��1 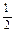 O2(g) =" CO" (g)
O2(g) =" CO" (g) 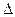 H1= ��110.5kJ/mol
H1= ��110.5kJ/mol