题目内容
一常见的液态碳氢化合物A,与溴水混合振荡,下层颜色变浅,上层变为棕红色.0.5mol A完全燃烧时,得到27g水和67.2L二氧化碳(标准状况).请回答下列问题.
(1)A的分子式
(2)A与液溴混合,在Fe的作用下发生反应生成B,写出反应的方程式 +Br2
+Br2
 +HBr
+HBr +Br2
+Br2
 +HBr.
+HBr.
(3)B与NaOH溶液反应的产物经酸化后得到有机物C,C的结构简式
 ,向C中加入饱和溴水,有
,向C中加入饱和溴水,有 +3Br2→
+3Br2→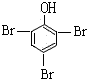 ↓+3HBr
↓+3HBr +3Br2→
+3Br2→ ↓+3HBr.
↓+3HBr.
(4)在催化剂作用下,C与甲醛反应得到一种高聚物,该高聚物的结构简式为
 .
.
(1)A的分子式
C6H6
C6H6
.(2)A与液溴混合,在Fe的作用下发生反应生成B,写出反应的方程式
 +Br2
+Br2| Fe |
 +HBr
+HBr +Br2
+Br2| Fe |
 +HBr
+HBr(3)B与NaOH溶液反应的产物经酸化后得到有机物C,C的结构简式


白色
白色
沉淀产生,反应的方程式为 +3Br2→
+3Br2→ ↓+3HBr
↓+3HBr +3Br2→
+3Br2→ ↓+3HBr
↓+3HBr(4)在催化剂作用下,C与甲醛反应得到一种高聚物,该高聚物的结构简式为


分析:27g水的物质的量=
=1.5mol,标况下67.2L二氧化碳的物质的量=
=3mol,故该烃分子中N(C)=
=6,N(H)=
=6,故该烃是分子式为C6H6,该烃与溴水混合振荡,下层颜色变浅,上层变为棕红色,溴被萃取,说明该烃分子中变化不饱和键,该烃为苯,据此解答.
| 27g |
| 18g/mol |
| 67.2L |
| 22.4L/mol |
| 3mol |
| 0.5mol |
| 1.5mol×2 |
| 0.5mol |
解答:解:(1)27g水的物质的量=
=1.5mol,标况下67.2L二氧化碳的物质的量=
=3mol,
故该烃分子中N(C)=
=6,N(H)=
=6,故该烃是分子式为C6H6,
故答案为:C6H6;
(2)该烃是分子式为C6H6,与溴水混合振荡,下层颜色变浅,上层变为棕红色,溴被萃取,说明该烃分子中变化不饱和键,故该烃为苯,苯与溴在Fe作催化剂条件下生成溴苯与HBr,反应方程式为: +Br2
+Br2
 +HBr,
+HBr,
故答案为: +Br2
+Br2
 +HBr;
+HBr;
(3)溴苯与NaOH溶液反应生成苯酚钠,经酸化后得到有机物C,C为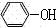 ;
;
向苯酚中加入饱和溴水,生成三溴苯酚白色沉淀,反应方程式为: +3Br2→
+3Br2→ ↓+3HBr,
↓+3HBr,
故答案为: ;白色;
;白色; +3Br2→
+3Br2→ ↓+3HBr;
↓+3HBr;
(4)在催化剂作用下,苯酚与甲醛反应得到一种高聚物,为酚醛树脂,该高聚物的结构简式为: ,
,
故答案为: .
.
| 27g |
| 18g/mol |
| 67.2L |
| 22.4L/mol |
故该烃分子中N(C)=
| 3mol |
| 0.5mol |
| 1.5mol×2 |
| 0.5mol |
故答案为:C6H6;
(2)该烃是分子式为C6H6,与溴水混合振荡,下层颜色变浅,上层变为棕红色,溴被萃取,说明该烃分子中变化不饱和键,故该烃为苯,苯与溴在Fe作催化剂条件下生成溴苯与HBr,反应方程式为:
 +Br2
+Br2| Fe |
 +HBr,
+HBr,故答案为:
 +Br2
+Br2| Fe |
 +HBr;
+HBr;(3)溴苯与NaOH溶液反应生成苯酚钠,经酸化后得到有机物C,C为
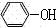 ;
;向苯酚中加入饱和溴水,生成三溴苯酚白色沉淀,反应方程式为:
 +3Br2→
+3Br2→ ↓+3HBr,
↓+3HBr,故答案为:
 ;白色;
;白色; +3Br2→
+3Br2→ ↓+3HBr;
↓+3HBr;(4)在催化剂作用下,苯酚与甲醛反应得到一种高聚物,为酚醛树脂,该高聚物的结构简式为:
 ,
,故答案为:
 .
.点评:本题考查有机物分子式与结构的确定、苯及苯酚的性质等,难度不大,计算确定有机物的分子式是解题的关键,注意有机物性质的掌握.

练习册系列答案
相关题目
