��Ŀ����
����Ŀ��ij��ѧС���Ʊ������������壺�ྻ��С�ձ�ʢ��������ˮ���þƾ��Ƽ��������ڣ����ձ�����μ���1 mol��L��1�Ȼ�����Һ����Һ������ĺ��ɫ���ش��������⣺
(1)����������������ֱ���ķ�Χ��________________��
(2)���齺���Ʊ��ɹ��IJ�����__________________________________��
(3)ʵ�����ֲ������������ƣ������Ȼ�����ҺӦ��ֹѪ����Ҫԭ����____________(�����)��
���Ȼ�����Һ����ɱ������������
���Ȼ�����Һ��ʹѪҺ��������
���Ȼ�����Һ�ܲ�����������������ס�˿�
���Ȼ�����Һ��ʹѪҺ������ѧ�仯
(4)�Ʊ�����Ļ�ѧ����ʽΪ_______�������1molFeCl3ȫ���Ƴɽ��壬������Ŀ______NA(ѡ����ڡ������ڡ���С�ڡ�)��
���𰸡�1��100nm ��һ�������䣬�۲쵽һ��������ͨ· �� FeCl3+3H2O ![]() Fe(OH)3(����)+3HCl С��
Fe(OH)3(����)+3HCl ��
��������
������������Һ�Ĺؼ����ڣ���������ֱ����С��1nm��100nm���ѱ����Ȼ�����Һ�����ˮ�м���������У��õ����ɫ��Һ��Ϊ�����������壬�������������ܲ��������ЧӦ�������м���������Һ����ʹ�����۳��������Ϸ������н��
��1���������������ǽ����ɢϵ�е�һ�֣���������ֱ����С��1nm��100nm��
�ʴ��ǣ�1nm��100nm��
��2��������������Һ�Ĺؼ����ڣ���Һ����ֱ��С��1nm����������ֱ����С��1nm��100nm������Һ�����������ЧӦ�����������������ܲ��������ЧӦ����һ���������������������壬�ܲ���һ��������ͨ·��
�ʴ��ǣ���һ���������䣬�ܲ���һ��������ͨ·��
��3��ѪҺ���ڽ��壬�Ȼ�����Һ���ڵ������Һ������������Һ��FeCl3��Һ����ʹ�����۳���ѪҺ���۴ﵽֹѪЧ����
�ʴ�ѡ�ڣ�
��4���ѱ����Ȼ�����Һ�����ˮ�м���������У��õ����ɫ��Һ��Ϊ�����������壬�Ʊ�����Ļ�ѧ����ʽΪ��FeCl3+3H2O ![]() Fe(OH)3(����)+3HCl�����ڽ����Ǵ������ӵļ����壬��������1molFeCl3ȫ���Ƴɽ��壬������ĿС��NA��
Fe(OH)3(����)+3HCl�����ڽ����Ǵ������ӵļ����壬��������1molFeCl3ȫ���Ƴɽ��壬������ĿС��NA��
�ʴ��ǣ�FeCl3+3H2O ![]() Fe(OH)3(����)+3HCl��С�ڡ�
Fe(OH)3(����)+3HCl��С�ڡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������V��Ϊ����Ԫ�أ����γɶ��̬�����ȫ��Һ�������һ�����͵���ɫ��������ϵͳ������ԭ������ͼ��

��֪��
�������� | VO2+ | VO2+ | V3+ | V2+ |
��ɫ | ��ɫ | ��ɫ | ��ɫ | ��ɫ |
��1��ȫ��Һ����طŵ�ʱV2+����������Ӧ���õ�طŵ�ʱ�ܷ�Ӧʽ��_______
��2������ɴ���ʱ��������Һ����ɫ�� __________
��3�����ӽ���Ĥ��������_________
��4��������ˮ�����ˮ����Ⱦ���Ժ�����ˮ����VO2+�⣬����Fe3+�ȣ������ۺϴ�����ʵ�ַ���Դ�Ļ������ã��������£�

��֪��Һ����Բ�ͬ��Ԫ�صĴ�����ʽ��ͬ��
���Ļ��ϼ� | ���� | ���� |
+4�� | VO2+ | VO(OH)3- |
+5�� | VO2+ | VO43- |
����Һ�з�Ԫ�ص���Ҫ������ʽΪ_______
�������ڿ������ɻҰ�ɫת��Ϊ���ɫ���û�ѧ�����ʾ����NaOH�����ɳ����ķ�Ӧ����_______________��____________��
����ȡ������ȡ��ʵ�ַ��ķ�����������̿ɼ�ʾΪ��HAΪ�л���ȡ������
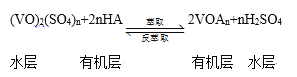
��ȡʱ��������������ԭ���� __________
�������������ε�ⷨ�������ַ��������ʣ���____����
����Ŀ��ʯ��ɽ�д�����ɽˮ�ֳ�����������о�NOx��SO2�ȴ�����Ⱦ������ƴ���������Ҫ���塣
(1)SO2���ŷ���Ҫ������ú��ȼ�գ���ҵ�ϳ��ð�ˮ���շ�����β���е�SO2��
��֪���չ�������ط�Ӧ���Ȼ�ѧ����ʽ���£�
��SO2(g)+NH3��H2O(aq)= NH4HSO3(aq) ��H1=a kJ/mol��
��NH3��H2O(aq)+ NH4HSO3(aq)=(NH4)2SO3(ag)+H2O(l) ��H2=b kJ/mol��
��2(NH4)2SO3(aq)+O2(g)=2(NH4)2SO4(aq) ��H3=c kJ/mol��
��Ӧ2SO2(g)+4NH3��H2O(aq)+O2(g) =2(NH4)2SO4(aq)+2H2O(l)����H=______kJ/mol��
(2)ȼú���糧�����÷�Ӧ2CaCO3(s)+2SO2(g)+O2(g)![]() 2CaSO4(s)+2CO2(g) ��H =681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪTKʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
2CaSO4(s)+2CO2(g) ��H =681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪTKʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
ʱ��/min Ũ��/mol��L1 | 0 | 10 | 20 | 30 | 40 | 50 |
O2 | 1.00 | 0.79 | 0.60 | 0.60 | 0.64 | 0.64 |
CO2 | 0 | 0.42 | 0.80 | 0.80 | 0.88 | 0.88 |
��0��10 min�ڣ�ƽ����Ӧ����v(SO2)=_____mol/(L��min)��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⡣�����ϱ��е������жϣ��ı������������_____������ĸ����
A��ͨ��һ������O2 B������һ�����ķ�״̼���
C���ʵ���С��������� D��������ʵĴ���
(3)NOx���ŷ���Ҫ����������β�����������÷�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��
N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��

��ͼ��֪��1050Kǰ��Ӧ��NO��ת�������¶����{��������ԭ��Ϊ_______����1100Kʱ��CO2���������Ϊ______��
(4)��ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��������Kp������1050K��1.1��106 Paʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=____[��֪�������ѹ(P��)=������ѹ(P)���������]��
(5)����β���������÷�Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H=746.8 kJ/mol��ʵ���ã�v��=k����c2(NO)��c2(CO)��v��=k����c(N2)��c2(CO2)��k����k��Ϊ���ʳ�����ֻ���¶��йأ���
N2(g)+2CO2(g) ��H=746.8 kJ/mol��ʵ���ã�v��=k����c2(NO)��c2(CO)��v��=k����c(N2)��c2(CO2)��k����k��Ϊ���ʳ�����ֻ���¶��йأ���
�ٴﵽƽ��������¶ȣ�k������ı���____���>����<����=����k������ı�����
������1L���ܱ������г���1 molCO��1 mol NO����һ���¶��´ﵽƽ��ʱ��CO��ת����Ϊ40%����k���Uk��=_____��



