��Ŀ����
10����������ڹ�˾�ٻ��˲���װ��DSG˫��ϱ�������������DSG˫��ϱ������������������������Ӽ���Ϊ���ͣ���ʻ�����п���ϵͳ�ġ���ᾧ���ᵼ�¶����жϺͷ������������½������仯������������������Ҳ������Ҫ����;����ش��������⣺��1������BP���͵�����BN�����ܵ��߶ȹ�ע����ĥͿ�ϣ����ǵĽṹ���ƣ���ͼΪ������ṹ����С���ظ���Ԫ������������廯������廯���������и��·�Ӧ�ϳɣ�

BBr3+PBr3+3H2=BP+6HBr��
�ش��������⣺
�ٻ�̬��ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p3��
������ľ���������ԭ�Ӿ��壮
��BBr3�����У�Bԭ�Ӳ�ȡsp2�ӻ�����BBr3���ڷǼ��ԣ�����ԡ��Ǽ��ԡ������ӣ�
�ܵ���������۵�Ҫ��������ߣ���ԭ���ǵ������������ԭ�Ӿ��壬ԭ�Ӿ����ۻ�ʱҪ�ƻ����ۼ��������ۼ��ļ����϶̡�����Խ��Խ�ι̣������۷е�Խ�ߣ���N�ıȽ�С��P�İ뾶������BN�����еļ������̡��۵���ߣ�
��2����ɰ��������������зֲ�����һ�֣������������������Ҳ���������Ƚⶾҩ����Ǻ��ᾧˮ���������ƣ���������Xm-����B��O��H����Ԫ�أ������ģ����ͼ��ʾ��
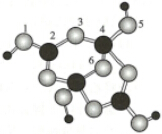
����Xm-�У���ԭ�ӹ�����ӻ�������sp2��sp3����λ��������4��5ԭ��֮�䣨��ԭ�ӵ����ֱ�ţ���m=2�������֣���
����ɰ������Na+��Xm-��H2O���ɣ�����֮����ڵ���������ADE������ţ���
A�����Ӽ� B�����ۼ� C�������� D�����»��� E�����
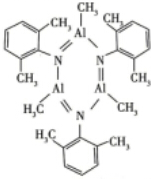
��3��B3N3H6�DZ��ĵȵ����壬�׳ơ���������������ͬ�壬Ҳ���γ����ƵIJ����������[Al��CH3��3]2��2��6-�����������п��ܺϳ����ƵIJ����д������Ľṹʽ
 ����Ӧ������ֻ���ͷų�һ��С����CH4��
����Ӧ������ֻ���ͷų�һ��С����CH4��
���� ��1����Pԭ�Ӻ�����15�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
�ڸ���ͼ֪��BP�Ĺ�������ԭ�ӣ����ݾ��幹����ȷ���������ͣ�
��BBr3�����У�Bԭ�Ӳ�ȡsp2�ӻ���˵���÷�����Bԭ�Ӽ۲���ӶԸ�����3����Ϊ��ԭ�Ӹ�����3������Bԭ�Ӳ����µ��Ӷԣ�Ϊƽ�������νṹ��������������غϵķ���Ϊ�Ǽ��Է��ӣ�
�ܵ������������ԭ�Ӿ��壬ԭ�Ӿ����۷е��뻯ѧ���ɷ��ȣ����ۼ��ļ���Խ�̡�����Խ��Խ�ι̣����۷е��Խ�ߣ�
��2���ٸ����γɻ�ѧ��֪����ɫС����Hԭ�ӡ���ɫ������Bԭ�ӡ���ɫ����Oԭ�ӣ�Bԭ�Ӽ۲���ӶԸ�����3��4���֣����ݼ۲���ӶԻ�������ȷ��Bԭ���ӻ����ͣ�����ͼ֪��4��5ԭ��֮���γ���λ�������������к���4��Hԭ�ӡ�4��Bԭ�ӡ�9��Oԭ�ӣ�1Ԫ�ػ��ϼ�Ϊ+1��BԪ�ػ��ϼ�Ϊ+3��OԪ�ػ��ϼ�Ϊ-2�����Ը������ϼ�=��-2����9+4����+3��+4����+1��=-2���ݴ�ȷ��mֵ��
�������Ӻ�������֮��������Ӽ���ˮ����֮����������ˮ�����д��ڹ��ۼ���
��3��B3N3H6�DZ��ĵȵ����壬�׳ơ���������������ͬ�壬Ҳ���γ����ƵIJ����������[Al��CH3��3]2��2��6-�����������п��ܺϳ����ƵIJ��˵��Al��Nԭ��֮���γ����Ʊ����ṹ���÷�Ӧ�൱��ȡ����Ӧ��ͬʱ���ɼ��飮
��� �⣺��1����Pԭ�Ӻ�����15�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p3���ʴ�Ϊ��1s22s22p63s23p3��
�ڸ���ͼ֪��BP�Ĺ�������ԭ�ӣ����ݾ��幹����֪���þ�����ԭ�Ӿ��壬�ʴ�Ϊ��ԭ�Ӿ��壻
��BBr3�����У�Bԭ�Ӳ�ȡsp2�ӻ���˵���÷�����Bԭ�Ӽ۲���ӶԸ�����3����Ϊ��ԭ�Ӹ�����3������Bԭ�Ӳ����µ��Ӷԣ�Ϊƽ�������νṹ���÷�����������������غϣ�����Ϊ�Ǽ��Է��ӣ��ʴ�Ϊ���Ǽ��ԣ�
�ܵ������������ԭ�Ӿ��壬ԭ�Ӿ����۷е��뻯ѧ���ɷ��ȣ����ۼ��ļ���Խ�̡�����Խ��Խ�ι̣����۷е��Խ�ߣ�Nԭ�Ӱ뾶С��Pԭ�ӣ�����N-B����С��P-B������N-B���ܴ��ι̣��������۷е�ߣ�
�ʴ�Ϊ���������������ԭ�Ӿ��壬ԭ�Ӿ����ۻ�ʱҪ�ƻ����ۼ��������ۼ��ļ����϶̡�����Խ��Խ�ι̣������۷е�Խ�ߣ���N�ıȽ�С��P�İ뾶������BN�����еļ������̡��۵���ߣ�
��2���ٸ����γɻ�ѧ��֪����ɫС����Hԭ�ӡ���ɫ������Bԭ�ӡ���ɫ����Oԭ�ӣ�Bԭ�Ӽ۲���ӶԸ�����3��4���֣����ݼ۲���ӶԻ�������֪Bԭ���ӻ�����Ϊsp2��sp3������ͼ֪��4��5ԭ��֮���γ���λ�������������к���4��Hԭ�ӡ�4��Bԭ�ӡ�9��Oԭ�ӣ�1Ԫ�ػ��ϼ�Ϊ+1��BԪ�ػ��ϼ�Ϊ+3��OԪ�ػ��ϼ�Ϊ-2�����Ը������ϼ�=��-2����9+4����+3��+4����+1��=-2������m=2��
�ʴ�Ϊ��sp2��sp3��4��5��2��
�������Ӻ�������֮��������Ӽ���ˮ����֮����������ˮ�����д��ڹ��ۼ�����ѡADE��
��3��B3N3H6�DZ��ĵȵ����壬�׳ơ���������������ͬ�壬Ҳ���γ����ƵIJ����������[Al��CH3��3]2��2��6-�����������п��ܺϳ����ƵIJ��˵��Al��Nԭ��֮���γ����Ʊ����ṹ���÷�Ӧ�൱��ȡ����Ӧ��ͬʱ���ɼ��飬�ò���ṹʽΪ ��
��
�ʴ�Ϊ�� ��CH4��
��CH4��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰���������жϡ�ԭ���ӻ���ʽ�жϡ���������Ų�ʽ����д��֪ʶ�㣬ͬʱ������ѧ����ѧ������֪ʶǨ����������Ϥ�۲���ӶԻ������ۣ�ע�⣨2��ģ���л�������λ�����ѵ��ǣ�3����ṹʽ����д��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ©�������������ձ�������̨����ֽ | B�� | �ձ����ƾ��ơ��Թܡ�©�� | ||
| C�� | ��ֽ���ձ����ԹܼС�©���������� | D�� | ����������ֽ����ƿ��©��������̨ |
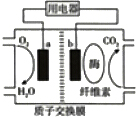 ������ṫ˾��ʾ��һ�ֵ�أ����Բ�����ֽ���У������ķ��������ˮ���õ�صĵ������Һ�л����ˮ��ø��ø�ֽ�ֽ�Ż���������ӣ��������������ϲ���ˮ���Ӷ��γɵ�������ṹʾ��ͼ��ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������
������ṫ˾��ʾ��һ�ֵ�أ����Բ�����ֽ���У������ķ��������ˮ���õ�صĵ������Һ�л����ˮ��ø��ø�ֽ�ֽ�Ż���������ӣ��������������ϲ���ˮ���Ӷ��γɵ�������ṹʾ��ͼ��ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������| A�� | �õ������ȼ�ϵ�� | |
| B�� | �õ�ؿ��ڸ�����ʹ�� | |
| C�� | aΪ�õ�صĸ��� | |
| D�� | b�������ĵ缫��ӦΪ����C6H10O5����+7nH2O+24ne-=6nCO2+24nH+ |
| 0�� | 50�� | 80�� | |
| Ca��OH��2 | 0.173g | 0.13g | 0.094g |
| Ba��OH��2•8H2O | 1.64g | 13.2g | 101.4g |

��1������۱�ΪCaO��BaO�Ļ����ͨ�����ý�̿��Ϊ��Դ���Ӿ���Ч��ĽǶȿ��ǣ�����̿�۸�������⣬����һ����Ҫԭ�������ý�̿ȼ�ղ����ĸ���ʹ��ʯ�ֽ⣬�йط�ӦΪC+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2��CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2����BaCO3$\frac{\underline{\;����\;}}{\;}$BaO+CO2����������Ӧ���ɵĶ�����̼��������������ȡ����CaCO3��BaCO3��ԭ�ϣ�
��2���Լ�a��ˮ�������ƣ���������ľ�����������ǽ����Һ������80�棬����Ca��OH��2�����˵õ�Ca��OH��2�����Ba��OH��2��Һ��
��3���Լ�b���Լ�c�Ƿ���ͬһ���ʣ�����ǡ�����д�������Լ�c������Ӧ�����ӷ���ʽ��CO2+Ba2++2OH-=BaCO3��+H2O��
| A�� | 20�� | B�� | 40�� | C�� | 48�� | D�� | 36�� |
| A�� | 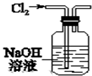 ��ͼװ�ó�ȥCl2�л��е�����HCl | |
| B�� |  ��ͼװ�÷���ˮ���屽�Ļ���� | |
| C�� |  ��ͼװ���Ʊ����ռ�NO���� | |
| D�� | 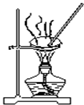 ��ͼװ������AlCl3������Һ�Ʊ�AlCl3���� |
 ��
��

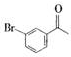 ��B
��B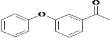 ��
�� ������ͬ���칹�壨���뺬�б������ʻ�����
������ͬ���칹�壨���뺬�б������ʻ�����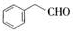 ��
��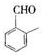 ��
��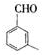 ��
�� ��
��