��Ŀ����
amol���������ʷֱ���������ϡ���ᷴӦ�����軹ԭ����ֻ��һ�֣���������������ʵ������ɿ��������֣�һ����Ϊ�������������ᣬ��һ����Ϊ����ģ�������������ӵ���ʽ�����ڷ�Ӧ�����Һ��ά����Һ�ĵ����ԣ������и�ѡ������ȷ���ǣ���λ��mol���� ��
��Fe��3a+a �� ��Fe3O4��9a+ ��
��Fe��OH��2��3a+ �� ��FeSO4��a+
A���٢ڢۢ� B��ֻ�Т٢� C��ֻ�Тۢ� D��ֻ�Т٢ڢ�
���𰸡�
A
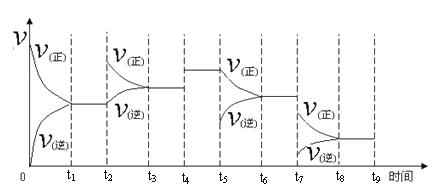
�������������������������������������������������õ����ᶼΪ��Ԫ�ص�3����A��B��C��D���������õ�����ֱ�Ϊ3amol��9amol��3amol��amol��A����������������Ϊamol��B��C��D���������������ᶼ��mol����ȫ����ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2NH3(g)����H =" -92.2" kJ��mol-1��
2NH3(g)����H =" -92.2" kJ��mol-1��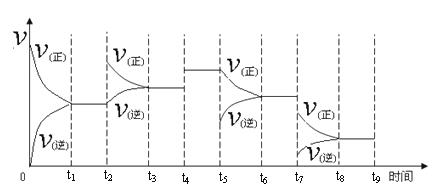
 H2(g) + CO(g) ��H =" +131.3" kJ/mol
H2(g) + CO(g) ��H =" +131.3" kJ/mol 2NH3(g)����H
= -92.2 kJ��mol-1��
2NH3(g)����H
= -92.2 kJ��mol-1��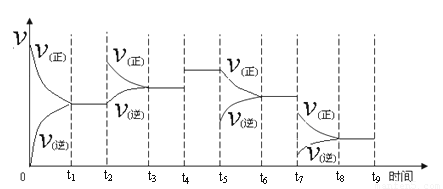
 H2(g)
+ CO(g) ��H = +131.3 kJ/mol
H2(g)
+ CO(g) ��H = +131.3 kJ/mol