��Ŀ����
(17��)ijѧ������ѧϰ�С����Խ̲���ͭ��Ũ���ᷴӦ��������о����ܹ���ͭ��Ӧ����������Ũ���Ƕ��٣��������⣬����������·�������ʵ�飺
ʵ���Լ���18mol/L����20mL����ͭ������������2mol/LNaOH��Һ
�����ʵ��ش����⣺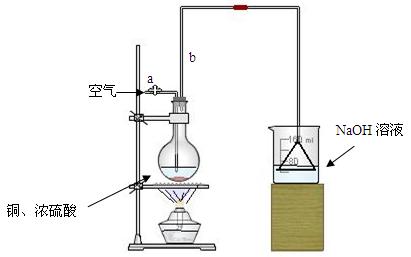
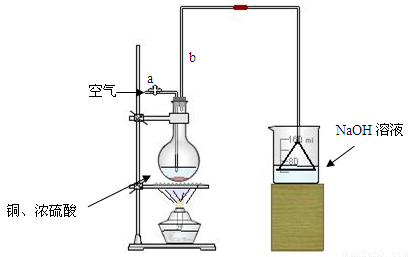
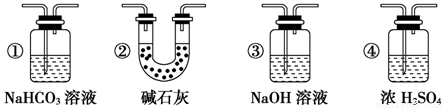
�����ȸ�����ͼ��ʾ����װʵ��װ�ã����ڼ����Լ�ǰ�Ƚ��� ������
���ձ�����NaOH��Һ���յ������ǣ� (�ѧʽ)�����õ��õ�©�������ǽ�������ֱ�������ձ��е�Ŀ���ǣ� ��
�Ǽ�����ƿ20���ӣ���ƿ�з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ������ƿ�з�Ӧ������������ȥ�ƾ��ƣ�������ƿ�е�����ʹ��Ӧ������ȫ��Ȼ���ɵ���aͨ�������Ŀ�������ȷ����ƿ�е�SO2����ȫ�������ձ��С��ڸ�ʵ��װ���е�
(����������)����ȷ������������ֲ�������á�
�Ƚ���ַ�Ӧ����ձ�ȡ�£������м����������ữ��˫��ˮ���ټ���������BaCl2��Һ���ٽ��� �� �� ��������ᱵ������Ϊ13.98g�����������ͭ��Ӧ�������Ũ������� ��
���е�ͬѧ�����������п��Բ��ؼ����ữ��˫��ˮ��ֱ�ӽ��к����ʵ�飬Ҳ�ܵõ�ȷ�����ݣ����������������Ƿ���Ҫ����˫��ˮ��ԭ��
��
�ż���װ�������� 2��
��SO2 1��
������ 2��
��Cu+2H2SO4=(��)=CuSO4+SO2��+2H2O 2��
������ 2��
�ȹ��ˡ�ϴ�ӡ����� ��1��
12mol/L 2��
����Ҫ��˫��ˮ 1��
ԭ�����ձ������ɵ�Na2SO3���ܱ���������ΪNa2SO4�����������˫��ˮ��ֱ�Ӳⶨ������������ȷ��SԪ�ص����ʵ������Ӷ�ʹʵ�������� 2��
����