��Ŀ����
����A��B��C�������ʣ�AΪ��̬�⻯�����ʽΪRH3����RΪ82.4%��B����һ����̬�⻯�A+B��C��C���Һ���ȷų�A��C��ˮ��Һ����ϡHNO3�ữ����AgNO3��Һ�����������Եİ�ɫ����������������⣺?��1��д��A�����ƺ͵���ʽ����ָ�����Ƿ��Ǽ��Է��ӣ����ȶ��Ա�PH3��H2Oǿ��������____________________________________________________________?
��2��д��B�����ƺ͵���ʽ����ָ�����Ƿ��Ǽ��Է��ӣ����ȶ��Ա�HF��H2S��HBr��Σ���ˮ��Һ�����Ա�HF��HBr��H2S��Σ�?__________________________________________????
��3��д��C�����ƺ͵���ʽ������ָ��C�����и����ֵĻ�ѧ����ָ��C�γɵľ������͡�__________________________________________?????
��4��д�������йصĻ�ѧ����ʽ�����ӷ���ʽ��?__________________________________________???
����������A�ķ���ʽ��R�ĺ�����֪��AΪNH3����![]() ,
,![]() )������C���Һ���ȷų�NH3����֪CΪ��Σ���BΪHCl��?
)������C���Һ���ȷų�NH3����֪CΪ��Σ���BΪHCl��?
�𰸣���1������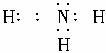 �����Է��ӡ��ȶ��ԣ�H2O��NH3��PH3��?
�����Է��ӡ��ȶ��ԣ�H2O��NH3��PH3��?
��2���Ȼ��⣬![]() �����Է��ӡ��ȶ��ԣ�HF��HCl��HBr��H2S������:HBr��HCl��H2S��HF��?
�����Է��ӡ��ȶ��ԣ�HF��HCl��HBr��H2S������:HBr��HCl��H2S��HF��?
��3���Ȼ�泥�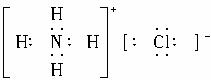 ��N��H���Լ���
��N��H���Լ���
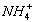 ��Cl-�γ����Ӽ������Ӿ��塣?
��Cl-�γ����Ӽ������Ӿ��塣?
��4��NH3+HCl=NH4Cl��NH4Cl+NaOH=NaCl+NH3��+H2O��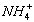 +OH-=NH3��+H2O�� NaCl+AgNO3=AgCl��+NaNO3��Cl-+Ag+=AgCl����
+OH-=NH3��+H2O�� NaCl+AgNO3=AgCl��+NaNO3��Cl-+Ag+=AgCl����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�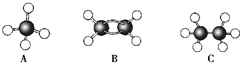
 �ɼ�дΪ
�ɼ�дΪ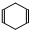 ������ij������W�ķ��ӽṹ�ɱ�ʾΪ��
������ij������W�ķ��ӽṹ�ɱ�ʾΪ��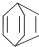 ��
��
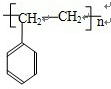

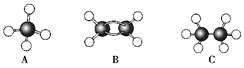
 �ɼ�дΪ
�ɼ�дΪ ������ij������W�ķ��ӽṹ�ɱ�ʾΪ��
������ij������W�ķ��ӽṹ�ɱ�ʾΪ�� ��
��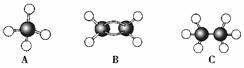

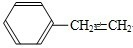
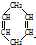 �ɼ�дΪ
�ɼ�дΪ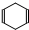 ������ij������W�ķ��ӽṹ�ɱ�ʾΪ��
������ij������W�ķ��ӽṹ�ɱ�ʾΪ��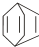 ��
��