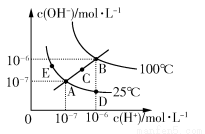
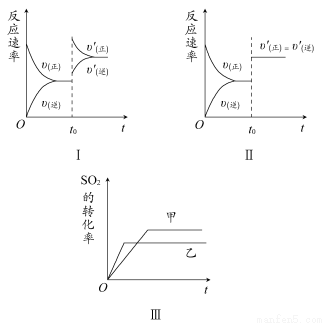
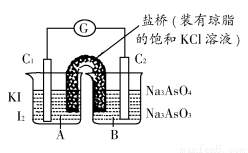
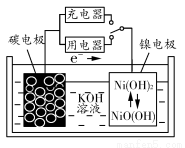
ЬтФПФкШн
ШчЭМЫљЪОЃЌгУЪЏФЋЕчМЋЕчНтТШЛЏЭШмвКЁЃВщдФзЪСЯПЩжЊЃЌCuCl42ЁЊЯдЛЦЩЋЃЌТШЛЏЭШмвКЯдРЖТЬЩЋЛђЛЦТЬЩЋЃЛЯђЬхЛ§ЯрЭЌХЈЖШЗжБ№ЮЊ0.01 mol/LЁЂ0.05 mol/LЁЂ0.1 mol/LЁЂ0.5 mol/LЕФТШЛЏЭШмвКжаМгШыNaClжСБЅКЭЃЌЖдБШЗЂЯжЃЌШмвКЕФбеЩЋгЩЛЦТЬЩЋЯђРЖТЬЩЋзЊБфЁЃ
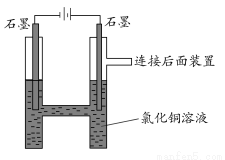
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)бєМЋЩЯЕФЦјЬхГЪ________ЩЋЃЌМьбщИУЦјЬхПЩгУ________________ЁЃ
(2)аДГіЕчНтЕФРызгЗНГЬЪНЃК________________ЁЃ
(3)ЪЕбщЪБЃЌзАжУжавѕМЋШмвКбеЩЋгЩРЖТЬЩЋБфЮЊЛЦТЬЩЋЃЌдвђЪЧ________ЁЃ
(4)ШЁГівѕМЋЕФЪЏФЋАєЃЌЗЂЯжБэУцгаЧГРЖЩЋЙЬЬхЃЌЪдЩшМЦЪЕбщЬНОПДЫЧГРЖЩЋЙЬЬхЕФГЩЗжЃК
____________________________________________________ЁЃ
(5)ЮЊЪВУДвЊЩшМЦСЌНгКѓУцЕФзАжУЃП
______________________________________________________________
(1)ЛЦТЬЩЋЁЁЪЊШѓЕФЕэЗлЃKIЪджНЁЁ(2)Cu2ЃЋЃЋ2ClЃ CuЃЋCl2Ёќ
CuЃЋCl2Ёќ
(3)вѕМЋCu2ЃЋЗХЕчКѓЃЌCu2ЃЋгыClЃХЈЖШБШжЕМѕаЁ
(4)ШЁДЫЧГРЖЩЋЙЬЬхгкЪдЙмжаЃЌМгШыСђЫсЃЌШєШмНтЧвШмвКБфРЖЩЋЃЌдђЮЊCu(OH)2
(5)вђЮЊбєМЋВњЩњЕФТШЦјЮЊгаЖОЦјЬхЃЌЙЪКѓУцСЌНгзАжУПЩвдгУгкМьбщгыЮќЪе
ЁОНтЮіЁП(1)бєМЋЪЧвѕРызгЗХЕчЃЌМДClЃЗХЕчВњЩњCl2ЃЌЙЪбеЩЋЮЊЛЦТЬЩЋЃЛМьбщCl2ПЩгУЪЊШѓЕФЕэЗлЃKIЪджНЃЌШєЪджНБфРЖЃЌдђШЗЖЈЮЊТШЦјЁЃ(2)ЕчНтТШЛЏЭЕФРызгЗНГЬЪНЮЊЃКCu2ЃЋЃЋ2ClЃ CuЃЋCl2ЁќЁЃ(3)гЩЬтФПЫљИјаХЯЂЃЌCu2ЃЋгыClЃХЈЖШБШжЕж№НЅдіДѓЃЌШмвКЕФбеЩЋгЩЛЦТЬЩЋЯђРЖТЬЩЋзЊБфЃЌЙЪвѕМЋCu2ЃЋЗХЕчКѓЃЌCu2ЃЋгыClЃХЈЖШБШжЕМѕаЁЖјГЪЯжЛЦТЬЩЋЁЃ(4)ЫцзХЗДгІЕФНјааЃЌCu2ЃЋХЈЖШНЕЕЭЃЌДЫЪБгавЛВПЗжHЃЋЗХЕчЖјЪЙДЫЧјгђOHЃХЈЖШдіДѓЃЌЙЪДЫЧГРЖЩЋЙЬЬхЮЊCu(OH)2ЁЃ(5)ЕчНтТШЛЏЭЪБВњЩњЕФТШЦјгаЖОЃЌЙЪКѓУцСЌНгзАжУМШПЩгУзїМьбщЕчНтВњЩњЕФЦјЬхЃЌгжПЩгУгкЮќЪеИУгаЖОЦјЬхЁЃ
CuЃЋCl2ЁќЁЃ(3)гЩЬтФПЫљИјаХЯЂЃЌCu2ЃЋгыClЃХЈЖШБШжЕж№НЅдіДѓЃЌШмвКЕФбеЩЋгЩЛЦТЬЩЋЯђРЖТЬЩЋзЊБфЃЌЙЪвѕМЋCu2ЃЋЗХЕчКѓЃЌCu2ЃЋгыClЃХЈЖШБШжЕМѕаЁЖјГЪЯжЛЦТЬЩЋЁЃ(4)ЫцзХЗДгІЕФНјааЃЌCu2ЃЋХЈЖШНЕЕЭЃЌДЫЪБгавЛВПЗжHЃЋЗХЕчЖјЪЙДЫЧјгђOHЃХЈЖШдіДѓЃЌЙЪДЫЧГРЖЩЋЙЬЬхЮЊCu(OH)2ЁЃ(5)ЕчНтТШЛЏЭЪБВњЩњЕФТШЦјгаЖОЃЌЙЪКѓУцСЌНгзАжУМШПЩгУзїМьбщЕчНтВњЩњЕФЦјЬхЃЌгжПЩгУгкЮќЪеИУгаЖОЦјЬхЁЃ

 ЦкФЉГхДЬ100ЗжДДаТН№ОэЭъШЋЪдОэЯЕСаД№АИ
ЦкФЉГхДЬ100ЗжДДаТН№ОэЭъШЋЪдОэЯЕСаД№АИ