��Ŀ����
����³���HCl����ƿ������Ȫʵ���õ�ϡ���ᣬ�ñ�̼������Һ�ζ���������ⶨ����ȷŨ�ȣ�����ش��������⣺
��1���ñ�̼������Һ�ζ���������ʱ��̼������ҺӦװ��______ʽ�ζ����У����ü�����ָʾ�����ﵽ�ζ��յ�ʱ����Һ��______ɫ���______ɫ��
��2������������Ũ�ȵı�̼������Һ������Ϊ����ʵ������е�______�֣�
��2.500mol/L ��0.25mol/L ��0.025mol/L
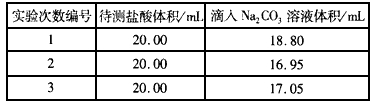
��3���������������ʵı�̼������Һ�ζ�����c��Na2CO3����ʾ���ζ�ʱʵ�������б����£�
�����ִ���ϡ��������ʵ���Ũ�ȣ����������������г�����ʽ���ɣ���c��HCl��=______��
��4����ʢװNa2CO3��Һ�ĵζ����ڵζ�ǰδ�ñ�Һ��ϴ���������������Ũ��______�����ζ���Ϻ����ʱ���ӣ���ʵ�����Ϊ______�����������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
��1���ñ�̼������Һ�ζ���������ʱ��̼������ҺӦװ��______ʽ�ζ����У����ü�����ָʾ�����ﵽ�ζ��յ�ʱ����Һ��______ɫ���______ɫ��
��2������������Ũ�ȵı�̼������Һ������Ϊ����ʵ������е�______�֣�
��2.500mol/L ��0.25mol/L ��0.025mol/L
��3���������������ʵı�̼������Һ�ζ�����c��Na2CO3����ʾ���ζ�ʱʵ�������б����£�
| ʵ�������� | �������������mL�� | ����̼������Һ�����mL�� |
| 1 | 20.00 | 18.80 |
| 2 | 20.00 | 16.95 |
| 3 | 20.00 | 17.05 |
��4����ʢװNa2CO3��Һ�ĵζ����ڵζ�ǰδ�ñ�Һ��ϴ���������������Ũ��______�����ζ���Ϻ����ʱ���ӣ���ʵ�����Ϊ______�����������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
��1��̼������Һ�ʼ��ԣ��ü�ʽ�ζ���ʢװ̼������Һ���ζ��յ�ʱ����Һ��ɫ�ɺ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ���ʴ�Ϊ����ʽ����ɫ����ɫ��
��2��HCl�Ĵ���Ũ��Ϊ
mol/L=0.045mol/L����̼������ҺӦѡ0.025mol/L����ѡ���ۣ�
��3����3����ȥ��һ�����ݣ�̼������Һ��ƽ�����Ϊ
��
2HCl��Na2CO3
2 1
C��HCl����20.00mL 0.025mol/L��
C(HCl)=
��
mol��L-1
�ʴ�Ϊ��C(HCl)=
��
mol��L-1��
��4��ʢװNa2CO3��Һ�ĵζ����ڵζ�ǰδ�ñ�Һ��ϴ����Һ��Ũ�Ƚ��ͣ����V����Һ��ƫ����c�����⣩=
��֪c�����⣩ƫ�ߣ��ζ���Ϻ����ʱ���ӣ����V����Һ��ƫС������c�����⣩=
��֪c�����⣩ƫС���ʴ�Ϊ��ƫ�ߣ�ƫ�ͣ�
��2��HCl�Ĵ���Ũ��Ϊ
| 1 |
| 22.4 |
��3����3����ȥ��һ�����ݣ�̼������Һ��ƽ�����Ϊ
| 16.95+17.05 |
| 2 |
2HCl��Na2CO3
2 1
C��HCl����20.00mL 0.025mol/L��
| 16.95+17.05 |
| 2 |
C(HCl)=
| 2��C(Na2CO3) |
| 20.00 |
| (16.95+17.05) |
| 2 |
�ʴ�Ϊ��C(HCl)=
| 2��C(Na2CO3) |
| 20.00 |
| (16.95+17.05) |
| 2 |
��4��ʢװNa2CO3��Һ�ĵζ����ڵζ�ǰδ�ñ�Һ��ϴ����Һ��Ũ�Ƚ��ͣ����V����Һ��ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |

��ϰ��ϵ�д�
�����Ŀ
����³���HCl����ƿ������Ȫʵ���õ�ϡ���ᣬ�ñ�̼������Һ�ζ���������ⶨ����ȷŨ�ȣ�����ش��������⣺
��1���ñ�̼������Һ�ζ���������ʱ��̼������ҺӦװ��______ʽ�ζ����У����ü�����ָʾ�����ﵽ�ζ��յ�ʱ����Һ��______ɫ���______ɫ��
��2������������Ũ�ȵı�̼������Һ������Ϊ����ʵ������е�______�֣�
��2.500mol/L ��0.25mol/L ��0.025mol/L
��3���������������ʵı�̼������Һ�ζ�����c��Na2CO3����ʾ���ζ�ʱʵ�������б����£�
�����ִ���ϡ��������ʵ���Ũ�ȣ����������������г�����ʽ���ɣ���c��HCl��=______��
��4����ʢװNa2CO3��Һ�ĵζ����ڵζ�ǰδ�ñ�Һ��ϴ���������������Ũ��______�����ζ���Ϻ����ʱ���ӣ���ʵ�����Ϊ______�����������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
��1���ñ�̼������Һ�ζ���������ʱ��̼������ҺӦװ��______ʽ�ζ����У����ü�����ָʾ�����ﵽ�ζ��յ�ʱ����Һ��______ɫ���______ɫ��
��2������������Ũ�ȵı�̼������Һ������Ϊ����ʵ������е�______�֣�
��2.500mol/L ��0.25mol/L ��0.025mol/L
��3���������������ʵı�̼������Һ�ζ�����c��Na2CO3����ʾ���ζ�ʱʵ�������б����£�
| ʵ�������� | �������������mL�� | ����̼������Һ�����mL�� |
| 1 | 20.00 | 18.80 |
| 2 | 20.00 | 16.95 |
| 3 | 20.00 | 17.05 |
��4����ʢװNa2CO3��Һ�ĵζ����ڵζ�ǰδ�ñ�Һ��ϴ���������������Ũ��______�����ζ���Ϻ����ʱ���ӣ���ʵ�����Ϊ______�����������ƫ�ߡ�����ƫ�͡�����Ӱ�족��