��Ŀ����
����Ŀ��2-����-3-�ȱ����ᣨF������Ҫ��ҽҩ�м��壬���Ʊ�����ͼ���£�
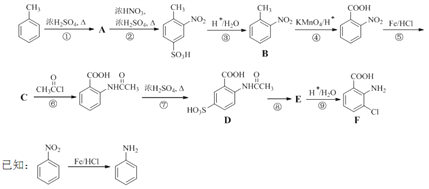
�ش��������⣺
��1��![]() �����в�ͬ��ѧ��������ԭ�ӹ���_______�֣�����ԭ����Ŀ���Ϊ_______��
�����в�ͬ��ѧ��������ԭ�ӹ���_______�֣�����ԭ����Ŀ���Ϊ_______��
��2��B������Ϊ_________��д��������������B������ͬ���칹��Ľṹ��ʽ_______��
a��������ֻ������ȡ�����һ�Ϊ��λ b�����ܷ���������Ӧ���ܷ���ˮ�ⷴӦ
��3��������δ���üױ�ֱ�������ķ����Ʊ�B�����Ǿ��ɢ٢ڢ�������Ӧ��ȡB����Ŀ����______________��
��4��д���Ļ�ѧ��Ӧ����ʽ��_________���ò���Ӧ����ҪĿ����____________��
��5��д����ķ�Ӧ�Լ���������_______________��F�к��������ŵ�����Ϊ__________��
��6���ڷ�����д���� Ϊ��Ҫԭ�ϣ������ٲ����Ʊ����ļ��ۺ�������̡�__________
Ϊ��Ҫԭ�ϣ������ٲ����Ʊ����ļ��ۺ�������̡�__________
 ����
����![]() Ŀ�껯����
Ŀ�껯����
���𰸡� 4 13 2-�����ױ����������ױ� 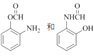 ���ⱽ���ϼ���λ����ԭ�ӱ�����ȡ��������ٸ������ռλ��
���ⱽ���ϼ���λ����ԭ�ӱ�����ȡ��������ٸ������ռλ�� 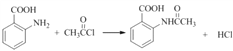 �������� Cl2/FeCl3����Cl2/Fe�� �Ȼ�
�������� Cl2/FeCl3����Cl2/Fe�� �Ȼ� 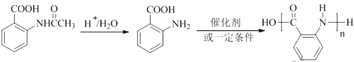
���������ױ���Ũ�������������ɶԼ������ᣬ������ᷢ��������Ӧ����Ӧ�۽������ˮ��ȥ����B��B�������Ը�����ط���������Ӧ�����������������Fe/HCl������ԭ��Ӧ��B�е�����ת��Ϊ��������CΪ�ڰ���������ٷ�����Ӧ�ޱ�����������Ӧ���������������Ȼ���һ����λλ��ռס����Ӧ���ȡ����Ӧ����Clԭ�ӣ���Ӧ���ٽ�����ת���������ɡ�
��1���ױ������в�ͬ��ѧ��������ԭ�ӹ���4�֣�����ԭ����Ŀ���Ϊ13����
��2��B������Ϊ�������ױ�����2-�����ױ�����
B��ͬ���칹�����ܷ���������Ӧ����ȩ�����������ṹ�����ܷ���ˮ�ⷴӦ��˵�����������ṹ�����ļ��ṹ���ɴ˿ɵ�B��ͬ���칹���У�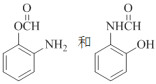 ��
��
��3�����ױ�ֱ�����������ø�����϶࣬�������ڼ��Ķ�λ����������Ӧ�����������������ױ��ȡ�
��4����Ӧ��Ϊ�ڰ����������������ȷ���ȡ����Ӧ������ʽΪ��
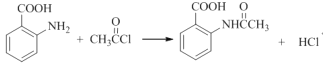 �����F�Ľṹ��֪���ò����Ŀ���DZ�����������ֹ������������
�����F�Ľṹ��֪���ò����Ŀ���DZ�����������ֹ������������
��5�����D��F�Ľṹ��֪����Ӧ������Clԭ�ӣ�����������Clԭ�ӵķ�������Fe����FeCl3����������������Cl2����ȡ����Ӧ��F�еĺ���������Ϊ�Ȼ���
��6�����ݲ���ἰ���۷�Ӧ�ɵ��������£�
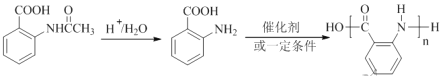 ��
��

����Ŀ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѡ���ش��������⣺
��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ��___________________________
��2��ú�����������в������к�����H2S��Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��______________________________________________
��3������ˮú���ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g)![]() CH3OH(g) ��H����90.8 kJ��mol-1
CH3OH(g) ��H����90.8 kJ��mol-1
�� 2CH3OH(g) ![]() CH3OCH3(g) + H2O(g) ��H����23.5 kJ��mol-1
CH3OCH3(g) + H2O(g) ��H����23.5 kJ��mol-1
�� CO(g) + H2O(g) ![]() CO2(g) + H2(g) ��H����41.3 kJ��mol-1
CO2(g) + H2(g) ��H����41.3 kJ��mol-1
�ܷ�Ӧ��3H2(g) + 3CO(g) ![]() CH3OCH3(g) +CO2 (g)�Ħ�H��__________ ��
CH3OCH3(g) +CO2 (g)�Ħ�H��__________ ��
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��___________(����ĸ����)
a�����¸�ѹ b��������� c������CO2��Ũ�� d������CO��Ũ�� e�������������
��4����֪��Ӧ��2CH3OH(g) ![]() CH3OCH3(g) + H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400 �����¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g) + H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400 �����¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/(mol��L-1) | 0.44 | 0.6 | 0.6 |
�ٱȽϴ�ʱ�����淴Ӧ���ʵĴ�С��v������ ______v���棩���>������<������)��
��������CH3OH��10 min��Ӧ�ﵽƽ�⣬��ʱc(CH3OH) �� _________����ʱ���ڷ�Ӧ����v(CH3OH) �� __________��