��Ŀ����
�����£�0.1 mol��L-1ijһԪ�ᣨHA����Һ��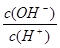 =1��10-8������������ȷ���ǣ� ��
=1��10-8������������ȷ���ǣ� ��
A����Һ��ˮ�������c(H+)��10-10 mol��L-1
B����Һ��c(H+)��c(A-)��0.1 mol��L-1
C����0.05 mol��L-1 NaOH��Һ�������Ϻ�������Һ������Ũ�ȴ�С��ϵΪ��c(A-)��c(Na+)��c(OH-)��c(H+)
D��������Һ�м���һ����CH3COONa������ˮϡ�ͣ���Һ��c(OH-)������
���𰸡�
D
��������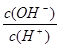 =1��10-8�������ˮ�����ӻ�������֪����Һ��������Ũ����10��3mol/L����˵���������ᡣ��Һ��OH����Ũ����10��11mol/L����Һ��ˮ�������c(H+)��10��11mol/L��A����ȷ��B����ȷ�����������غ�Ӧ���� c(HA)��c(A-)��0.1 mol��L-1�� ���ݵ���غ��֪c(A-)��c(OH-)��c(Na+)��c(H+)������ѡ��C�Dz���ȷ�ģ������ȷ�Ĵ�ѡD��
=1��10-8�������ˮ�����ӻ�������֪����Һ��������Ũ����10��3mol/L����˵���������ᡣ��Һ��OH����Ũ����10��11mol/L����Һ��ˮ�������c(H+)��10��11mol/L��A����ȷ��B����ȷ�����������غ�Ӧ���� c(HA)��c(A-)��0.1 mol��L-1�� ���ݵ���غ��֪c(A-)��c(OH-)��c(Na+)��c(H+)������ѡ��C�Dz���ȷ�ģ������ȷ�Ĵ�ѡD��

��ϰ��ϵ�д�
�����Ŀ