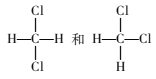
��Ŀ����
����Ŀ����NAΪ�����ӵ���������ֵ�������й�������ȷ���ǣ� ��
A. 7.8gNa2O2�������������ĵ�����Ϊ1.8NA
B. ��״���£�22.4LCCl4������C��C1������ĿΪ4NA
C. �ں�Al3+����ΪNA��AlCl3��Һ�У�Cl-����Ϊ3NA
D. ��11P4+60CuSO4+96H2O=20Cu3P+24H3PO4+60H2SO4��Ӧ�У�6molCuSO4���������ķ�����Ϊ1.1NA
���𰸡�A
��������A. 7.8gNa2O2�����ʵ�����0.1mol��������������O22���������ĵ�����Ϊ1.8NA��A��ȷ��B. ��״�������Ȼ�̼��Һ�壬������������Ħ���������22.4LCCl4������C��C1������Ŀ��B����C. ����������Һ��ˮ�⣬����ں�Al3+����ΪNA��AlCl3��Һ�У�Cl-��������3NA��C����D. CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�CuSO4����������P4������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���Ԫ�صĻ��ϼۼ������ֽ��ͣ�����P4�������������ǻ�ԭ��������11molP4�μӷ�Ӧ������5mol��P4����������60mol����ͭ����������ֻ��6mol��P4����ԭ�������ɵ����غ��֪����6 mol��CuSO4�μӷ�Ӧ��������ͭ�����İ����ӵ����ʵ���Ϊ![]() ��D����ѡA��
��D����ѡA��

����Ŀ�����ײ���һֱ�������о�����Ҫ���⣬��������Fe�۱�������г�ǿ�Ĵ��ԣ���Ч���Ե����������ʡ�
I��ʵ���Ҳ������ԭ���Ʊ�����Fe����������ͼ��ʾ��

��1������Fe��ϡ���ᷴӦ�����ӷ���ʽΪ_______________________________��
��2����ν�FeCl2��nH2O���������ˮ�Ƶ���ˮFeCl2 _____________________________________(�ü�Ҫ��������)��
��3����������Fe�Ļ�ѧ����ʽΪ______________________________________��
II���������ϣ��ڲ�ͬ�¶��£�����Fe����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570��ʱ����FeO������570��ʱ����Fe3O4����ͬѧ����ͼ��װ����ʾ��������Fe����ˮ������Ӧ��ʵ�飬��ͬѧ��ͼ����ʾ��װ�ý�������Fe����ˮ�����ķ�Ӧ����֤���
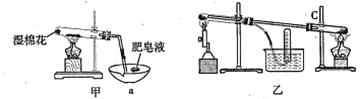
��4����װ��������Fe����ˮ������Ӧ�Ļ�ѧ����ʽ�� ______________________��
��5����װ��������a������Ϊ_______________________��
��6����ͬѧΪ̽��ʵ��������Թ��ڵĹ������ʳɷ֣�����������ʵ�飺
ʵ�鲽�� | ʵ����� | ʵ������ |
I | ����Ӧ��õ��ĺ�ɫ��ĩX(�ٶ�Ϊ���ȵ�)��ȡ������������һ�Թ��У������������ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ��dz��ɫ�����������ݲ��� |
II | ��ʵ��I�õ�����Һ�еμӼ���KSCN��Һ���� | ��Һû�г��ֺ�ɫ |
��������ʵ�飬��ͬѧ��Ϊ�������·�Ӧ�Ĺ������ΪFeO��
��ͬѧ��Ϊ��ͬѧ�Ľ��۲���ȷ������������______(�ü�Ҫ��������)��
��7����ͬѧ��ȡ5.60gFe�ۣ�����װ��Ӧһ��ʱ���ֹͣ���ȡ����Թ��ڵĹ��������ڸ���������ȴ�Ƶ�����Ϊ6.88g����ͬѧʵ���Ĺ������������������������Ϊ________(���������λ��Ч����)��