��Ŀ����
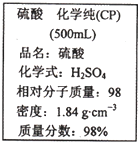
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol?L-1��ϡ���ᣮ
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol?L-1��ϡ���ᣮ
�ɹ�ѡ�õ������У�
�ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
��1��ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�����о�ʾ����е�______

��2������ϡ����ʱ����ȱ�ٵ�������______��д�������ƣ���
��3�������㣬����100mL1mol?L-1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ______mL����ȡ����ʱӦѡ��______������Ͳ��
A��10mL����B��50mL���� C��100mL����D��200mL
��4�����ձ���ϡ��Ũ�����ʵ�����Ϊ______������ϡ�����У�����Ũ�����մ�����ϣ���������Ϊ______��
��5���������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1mol?L-1�����ƹ��������и�������������������ԭ��______��
A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B������ƿ������ˮϴ�Ӻ�δ���������������ˮ
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D��ת����Һʱ��������������Һ��������ƿ����
E������ʱ����������ƿ�̶��߽��ж���
F�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���
�⣺��1��Ũ�������ǿ�ҵĸ�ʴ�ԣ�����Ӧ��ǩ��Ӧӡ�и�ʴƷ��־��
��ѡ��D��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���ṩ��������֪����Ҫ�����У�100mL����ƿ����������
��3��ŨH2SO4�����ʵ���Ũ��c= mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��
����xmL��18.4mol/L=100mL��1mol/L����ã�x��5.4��
����Ũ��������Ϊ5.4mL����Ͳ���Խ�ӽ�����Ũ����������ԽС������ѡ��10mL��Ͳ��
�ʴ�Ϊ��5.4��A��
��4��Ũ����ϡ�Ͳ���Ϊ��Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裻
����Ũ�����մ�����ϣ���������Ϊ��������Ĩ����ȥ���ô���ˮ��ϴ��Ϳ��3%��5%NaHCO3��ϡ��Һ��
�ʴ�Ϊ����Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裻
������Ĩ����ȥ���ô���ˮ��ϴ��Ϳ��3%��5%NaHCO3��ϡ��Һ��
��5��A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ��ȡŨ������������������ҺŨ��ƫ�ߣ�
B�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C����Һ���������������ʣ�Ũ����ϡ�ͣ��ų��������ȣ��ܽ��δ�ָ���������ת�Ƶ�����ƿ�ж��ݣ�����������Һ�����С��������ҺŨ��ƫ�ߣ�
D��ת����Һʱ��������������Һ��������ƿ���棬��������ƿ�е�������������ʵ�����С��������ҺŨ��ƫ�ͣ�
E������ʱ����������ƿ�̶��ߣ�����������Һ�����С��������ҺŨ��ƫ�ߣ�
F��ҡ�Ⱥ�Һ���½���һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ�
��ѡ��ACE��
��������1��Ũ�������ǿ�ҵĸ�ʴ�ԣ�
��2������������Һ��ʵ���������ѡ�������������
��3������c= ����ŨH2SO4�����ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
����ŨH2SO4�����ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
��������Ũ��������ѡ����Ͳ���
��4��Ũ����ϡ�Ͳ���Ϊ��Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裻
������Ĩ����ȥ���ô���ˮ��ϴ��Ϳ��3%��5%NaHCO3��ϡ��Һ��
����5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=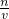 �����жϣ�
�����жϣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=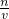 ��������ԭ����ע��Ũ�����ϡ�ͣ�
��������ԭ����ע��Ũ�����ϡ�ͣ�
��ѡ��D��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���ṩ��������֪����Ҫ�����У�100mL����ƿ����������
��3��ŨH2SO4�����ʵ���Ũ��c=
 mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=100mL��1mol/L����ã�x��5.4��
����Ũ��������Ϊ5.4mL����Ͳ���Խ�ӽ�����Ũ����������ԽС������ѡ��10mL��Ͳ��
�ʴ�Ϊ��5.4��A��
��4��Ũ����ϡ�Ͳ���Ϊ��Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裻
����Ũ�����մ�����ϣ���������Ϊ��������Ĩ����ȥ���ô���ˮ��ϴ��Ϳ��3%��5%NaHCO3��ϡ��Һ��
�ʴ�Ϊ����Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裻
������Ĩ����ȥ���ô���ˮ��ϴ��Ϳ��3%��5%NaHCO3��ϡ��Һ��
��5��A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ��ȡŨ������������������ҺŨ��ƫ�ߣ�
B�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C����Һ���������������ʣ�Ũ����ϡ�ͣ��ų��������ȣ��ܽ��δ�ָ���������ת�Ƶ�����ƿ�ж��ݣ�����������Һ�����С��������ҺŨ��ƫ�ߣ�
D��ת����Һʱ��������������Һ��������ƿ���棬��������ƿ�е�������������ʵ�����С��������ҺŨ��ƫ�ͣ�
E������ʱ����������ƿ�̶��ߣ�����������Һ�����С��������ҺŨ��ƫ�ߣ�
F��ҡ�Ⱥ�Һ���½���һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ�
��ѡ��ACE��
��������1��Ũ�������ǿ�ҵĸ�ʴ�ԣ�
��2������������Һ��ʵ���������ѡ�������������
��3������c=
 ����ŨH2SO4�����ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
����ŨH2SO4�����ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ��������������Ũ������������������Ũ��������ѡ����Ͳ���
��4��Ũ����ϡ�Ͳ���Ϊ��Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裻
������Ĩ����ȥ���ô���ˮ��ϴ��Ϳ��3%��5%NaHCO3��ϡ��Һ��
����5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
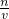 �����жϣ�
�����жϣ����������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
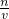 ��������ԭ����ע��Ũ�����ϡ�ͣ�
��������ԭ����ע��Ũ�����ϡ�ͣ� 
��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ�����˵��������ǣ�������
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ�����˵��������ǣ�������| A�����������ǿ�ҵĸ�ʴ�ԣ�Ӧ����Σ�ջ�ѧ��Ʒ�������Ʊ��� | B��ȡ10 mL���������ձ��У��ټӵ������ˮ�������49%������ | C������200mL4.6 mol?L-1��ϡ������ȡ������50mL | D�����������������ˮ���������Һ�����ʵ���Ũ��С��9.2 mol?L-1 |
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ�����˵����ȷ���ǣ�������
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ�����˵����ȷ���ǣ�������
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ�����˵������ȷ���ǣ�������
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ�����˵������ȷ���ǣ������� ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol?L-1��ϡ���ᣮ
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol?L-1��ϡ���ᣮ