��Ŀ����
ʵ��������Na2CO3�q10H2O��������0.4mol/L��Na2CO3��Һ250mL��
��1��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ
��2��Ҫ��ɱ�ʵ����
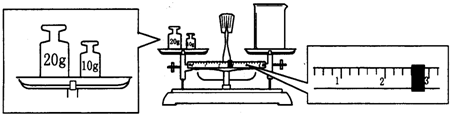
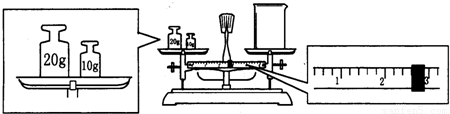
ijͬѧ����Na2CO3�q10H2O�����ձ��г�����������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ

��3��ʹ������ƿǰ������е�һ��������
��4�������ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���
��û��ϴ���ձ��Ͳ�����
��ת����Һʱ������������������ƿ����
������ƿ�����������������ˮ
�ܶ���ʱ���ӿ̶���
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
��1��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ
�ڢ٢ۢ�ݢޢߢ�
�ڢ٢ۢ�ݢޢߢ�
����ʵ������õ�����������ƽ��ҩ�ס����������ձ�250mL����ƿ����ͷ�ι�
250mL����ƿ����ͷ�ι�
����2��Ҫ��ɱ�ʵ����
28.6
28.6
gNa2CO3�q10H2O���壮ijͬѧ����Na2CO3�q10H2O�����ձ��г�����������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ
27.4
27.4
g��
��3��ʹ������ƿǰ������е�һ��������
��©
��©
����4�������ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���
�ܢ�
�ܢ�
����û��ϴ���ձ��Ͳ�����
��ת����Һʱ������������������ƿ����
������ƿ�����������������ˮ
�ܶ���ʱ���ӿ̶���
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
��������1������ʵ������IJ����ʹ�õ�������ɣ�
��2������n=cv��������Na2CO3�����ʵ���������Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3?10H2O����������ƽƽ��ԭ��Ϊ����������=����������+������ֵ���ݴ˼��㣻
��3���������ƿ�Ƿ�©ˮ��
��4������c=
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��2������n=cv��������Na2CO3�����ʵ���������Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3?10H2O����������ƽƽ��ԭ��Ϊ����������=����������+������ֵ���ݴ˼��㣻
��3���������ƿ�Ƿ�©ˮ��
��4������c=
| n |
| V |
����⣺��1�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ˳��Ϊ�ڢ٢ۢ�ݢޢߢܣ�����Ҫ�õ�250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�250mL����ƿ����ͷ�ιܣ�
��2����������Һ�����Ϊ240ml��������ƿ�Ĺ��û��240ml��ֻ��ѡ��250ml��Na2CO3�����ʵ���n=cV=0.25L��0.4mol?L-1=0.1mol��Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3?10H2O������0.1mol��286g/mol=28.6g����ƽƽ��ԭ��Ϊ����������=����������+������ֵ������ʵ�ʳƵ�̼���ƾ��������Ϊ30g-2.6g=27.4g��
�ʴ�Ϊ��28.6��27.4g��
��3��ʹ������ƿǰ������е�һ�������Ǽ������ƿ�Ƿ�©ˮ��
�ʴ��ǣ���©��
��4����û��ϴ���ձ��Ͳ������������������ʵ������٣�Ũ��ƫС���ʢٴ���
��ת����Һʱ������������������ƿ���棬�������ʵ����ʵ���ƫС��Ũ��ƫС���ʢڴ���
������ƿ�����������������ˮ����Ӱ�����ƽ�����ʢ۴���
�ܶ���ʱ���ӿ̶��ߣ�����������Һ�����ƫС��Ũ��ƫ�ߣ��ʢ���ȷ��
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ��������Ƶ���Һ�����ƫС��Ũ��ƫ�ߣ��ʢ���ȷ��
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�����������Һ�����ƫ��Ũ��ƫ�ͣ��ʢ���
ƫ�ߵ��Тܢݣ�
��ѡ�ܢݣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�250mL����ƿ����ͷ�ιܣ�
��2����������Һ�����Ϊ240ml��������ƿ�Ĺ��û��240ml��ֻ��ѡ��250ml��Na2CO3�����ʵ���n=cV=0.25L��0.4mol?L-1=0.1mol��Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3?10H2O������0.1mol��286g/mol=28.6g����ƽƽ��ԭ��Ϊ����������=����������+������ֵ������ʵ�ʳƵ�̼���ƾ��������Ϊ30g-2.6g=27.4g��
�ʴ�Ϊ��28.6��27.4g��
��3��ʹ������ƿǰ������е�һ�������Ǽ������ƿ�Ƿ�©ˮ��
�ʴ��ǣ���©��
��4����û��ϴ���ձ��Ͳ������������������ʵ������٣�Ũ��ƫС���ʢٴ���
��ת����Һʱ������������������ƿ���棬�������ʵ����ʵ���ƫС��Ũ��ƫС���ʢڴ���
������ƿ�����������������ˮ����Ӱ�����ƽ�����ʢ۴���
�ܶ���ʱ���ӿ̶��ߣ�����������Һ�����ƫС��Ũ��ƫ�ߣ��ʢ���ȷ��
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ��������Ƶ���Һ�����ƫС��Ũ��ƫ�ߣ��ʢ���ȷ��
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�����������Һ�����ƫ��Ũ��ƫ�ͣ��ʢ���
ƫ�ߵ��Тܢݣ�
��ѡ�ܢݣ�
���������⿼����һ�����ʵ���Ũ����Һ�������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע�����

��ϰ��ϵ�д�
�����Ŀ