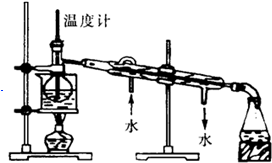
��Ŀ����
13��ij��ѧ����С��Ϊ�ⶨ������CO2�ĺ���������������ʵ�飺������0.1000mol/L��0.0100mol/L�ı����ᣮ
����0.1000mol/L�ı�����ζ�δ֪Ũ�ȵ�Ba��OH��2��Һ10.00mL�������ȥ����19.60mL��
���òⶨ��Ba��OH��2��Һ���ն��������е�CO2��ȡBa��OH��2��Һ10.00mL����100mL����ƿ���ˮ���̶��ߣ�ȡ��ϡ�ͺ����Һ�����ܱ������ڣ���ͨ��10L��״���µĿ�������ʱ���ɳ�����
�ܹ�������������Һ��
��ȡ��Һ20.00mL����0.0100mol/L������ζ�����ȥ����34.80mL����ش��������⣺
��1�����Ʊ�����ʱ������������Щ������B��C��D��E��F��G��
A��������ƽ��B������ƿ��C����ʽ�ζ��ܣ�D����Ͳ��E���ձ��� F����ͷ�ιܣ� G����������
��2���ζ������У����ֿ��ƻ����أ�����ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��
��3��Ba��OH��2��Һ�����ʵ���Ũ����0.0980mol/L��
��4������������Һ��Ŀ���Ƿ����BaCO3����ֹHCl��BaCO3��Ӧ��
��5���˿�����Ʒ�к�CO2���������Ϊ0.025%��
��6����ʵ���У�����һ�εζ�ʱʹ�õ���ʽ�ζ���δ����������ע��ڶ��ֱ����ᣬ�����еڶ��εζ���ʹ�ⶨ���ƫ���ƫ��ƫС������Ӱ�족����
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��������
��2��������ȷ�ĵζ�ʵ�����������ɣ�
��3�����ݷ�Ӧ����ʽ���ζ����ݽ��м��㣻
��4�������̼�ᱵ����ֹ̼�ᱵ�����ᷴӦ��Ӱ��ζ������
��5�����������̼��Ӧ��ʣ���Ba��OH��2������Ba��OH��2�����ʵ��������������CO2�����ʵ��������������
��6���ڶ��εζ�ʱ����ʽ�ζ���û����ϴ�����±�Һ�����Ũ��ƫ�ͣ����ĵ���������ƫС���������ʣ��������������ʵ���ƫС��
��� �⣺��1������һ�����ʵ���Ũ�ȵ�����ʱ�������м��١�ϡ�͡�ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫ�����У�����ƿ����Ͳ��������ʽ�ζ��ܣ����ձ�����ͷ�ιܼ�����������BCDEFG��
�ʴ�Ϊ��BCDEFG��
��2���ζ�����ʱ�����ֿ��ƻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��
�ʴ�Ϊ�����ƻ����� ҡ����ƿ��ע����ƿ����Һ��ɫ�ı仯��
��3�����ݷ�Ӧ����ʽ��Ba��OH��2+2HCl�TBaCl2+2H2O����c[Ba��OH��2]��10.00 mL=$\frac{1}{2}$��0.1 mol/L��19.60 mL�����c[Ba��OH��2]=0.098 mol/L��
�ʴ�Ϊ��0.098 mol/L��
��4����һ����Ҫ������ζ��������̼��Ӧ��ʣ�������������Ϊ�˷�ֹBaCO3��HCl������Ӧ��Ӱ��ζ������Ӧ�÷����BaCO3��
�ʴ�Ϊ�������BaCO3����ֹHCl��BaCO3��Ӧ��
��5�������ķ�Ӧ����ʽ��Ba��OH��2+CO2�TBaCO3��+H2O��20 mL��Һ��Ba��OH��2�����ʵ���Ϊ34.80��10-3��0.01��$\frac{1}{2}$mol=0.174��10-3 mol����ô100 mL��Һ����Ba��OH��2��100mL��Һ�к��е��������������ʵ���Ϊ��0.174��10-3��$\frac{100ml}{20ml}$mol=8.70��10-4 mol��
ԭ�е��������������ʵ���Ϊ��0.098 mol/L��10��10-3L=9.8��10-4 mol��
�����ж�����̼���ĵ��������������ʵ���Ϊ��9.8��10-4 mol-8.70��10-4 mol=1.1��10-4 mol��n��CO2��=n��Ba��OH��2��=1.1��10-4 mol��
������̼���������Ϊ��$\frac{1.1��1{0}^{-4}��22.4}{10}$��100%=0.025%��
�ʴ�Ϊ��0.025%��
��6��������ʽ�ζ���û����ϴ�����µڶ��εζ����ĵı�Һ�������ƫС��������������������ʵ���ƫС�����������̼��Ӧ�������������ʵ���ƫ������ƫ��
�ʴ�Ϊ��ƫ��
���� ���⿼���˲ⶨ������CO2�ĺ������漰��������Һ������ѡ����������ļ��㡢ʵ�����������֪ʶ����ֿ�����ѧ���ķ�������������������ѧ֪ʶ����������������Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� |  �ձ� | B�� |  ��ƿ | C�� |  ����ƿ | D�� |  ��ͷ�ι� |
| A�� | �� | B�� | �� | C�� | �� | D�� | ������ |
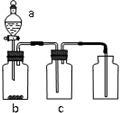 ����ͼװ����ȡ���ᴿ���ռ����е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е��ǣ�������
����ͼװ����ȡ���ᴿ���ռ����е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е��ǣ�������| ���� | a | b | c | |
| A | NO2 | Ũ���� | ͭƬ | NaOH��Һ |
| B | SO2 | Ũ���� | Cu | Ũ���� |
| C | O2 | ˫��ˮ | �������� | ��ʯ�� |
| D | CO2 | ϡ���� | CaCO3 | ����NaHCO3��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��������ƽȷ��ȡ0.400 0 gʳ�Σ����500 mLŨ��Ϊ0.020 0 mol•L-1��ʳ��ˮ | |
| B�� | ����ˮ�͵�ˮ�зֱ��������CCl4�����ã��²����ɫ�����ϲ��� | |
| C�� | �ù�����NaOH��Һ��ȥFeCl2��Һ�е�FeCl3 | |
| D�� | ij������ʹƷ����Һ��ɫ���������һ����SO2 |
| A�� | pH=11����Һ�У�CO32-��Na+��AlO2-NO3- | |
| B�� | ��ɫ��Һ�У�K+��Na+��MnO4-��SO42- | |
| C�� | ����Al�ܷų�H2����Һ�У�Cl-��HCO3-��SO42-��NH4+ | |
| D�� | ������Һ�У�Fe2+��Al3+��NO3-��Cl- |