��Ŀ����
�ܱ������г���10 mol CO��20 mol H2���ڴ��������·�Ӧ���ɼ״���CO(g)��2H2(g) CH3OH(g)��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ� ��
CH3OH(g)��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ� ��
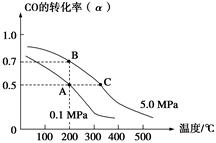
A����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱA��B����ʱ�����У�n(A)����n(B)����4��5
B����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA��tC
C����B��C�����ƽ�ⳣ���ֱ�ΪKB��KC����KB��KC
D���ڲ��ı䷴Ӧ������������£����¡���ѹ�����״��ӻ����ϵ�з�������������CO��ת����
 CH3OH(g)��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ� ��
CH3OH(g)��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ� ��
A����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱA��B����ʱ�����У�n(A)����n(B)����4��5
B����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA��tC
C����B��C�����ƽ�ⳣ���ֱ�ΪKB��KC����KB��KC
D���ڲ��ı䷴Ӧ������������£����¡���ѹ�����״��ӻ����ϵ�з�������������CO��ת����
D
�� CO(g)��2H2(g)  CH3OH(g)
CH3OH(g)
��ʼ/mol 10 20 0
Aƽ��/mol 5 10 5
Bƽ��/mol 3 6 7
ѡ��A�У�n(A)����n(B)����20��16��5��4��ѡ��A����A���C����ȣ�C����¶ȸߡ�ѹǿ��Ӧ���ʿ죬����ƽ���ʱ��̣�tA��tB��ѡ��B����B��C�㣬�¶����ߣ�ƽ�����ƣ�K��С����KB��KC��ѡ��C�����¡���ѹ������������Ũ�ȶ�ʹƽ�����ƣ�CO��ת��������ѡ��D��ȷ��
 CH3OH(g)
CH3OH(g)��ʼ/mol 10 20 0
Aƽ��/mol 5 10 5
Bƽ��/mol 3 6 7
ѡ��A�У�n(A)����n(B)����20��16��5��4��ѡ��A����A���C����ȣ�C����¶ȸߡ�ѹǿ��Ӧ���ʿ죬����ƽ���ʱ��̣�tA��tB��ѡ��B����B��C�㣬�¶����ߣ�ƽ�����ƣ�K��С����KB��KC��ѡ��C�����¡���ѹ������������Ũ�ȶ�ʹƽ�����ƣ�CO��ת��������ѡ��D��ȷ��

��ϰ��ϵ�д�
�����Ŀ
 bY(g)��cZ(g)�ﵽƽ��״̬�����������ѹ����ԭ����1/2���Ҵﵽ�µ�ƽ��״̬ʱ��X�����ʵ���Ũ�ȴ�0.1 mol/L����0.19 mol/L�������ж���ȷ����(����)
bY(g)��cZ(g)�ﵽƽ��״̬�����������ѹ����ԭ����1/2���Ҵﵽ�µ�ƽ��״̬ʱ��X�����ʵ���Ũ�ȴ�0.1 mol/L����0.19 mol/L�������ж���ȷ����(����) CH3OH(g)�������ݻ���Ϊ1 L��a��b��c�����ܱ������зֱ����1 mol CO��2 mol H2�Ļ�����壬�����¶Ƚ��з�Ӧ�����������ݵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
CH3OH(g)�������ݻ���Ϊ1 L��a��b��c�����ܱ������зֱ����1 mol CO��2 mol H2�Ļ�����壬�����¶Ƚ��з�Ӧ�����������ݵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
 3C(g)+ D(g)���ݻ�������ܱ������н���,�ﵽ��ѧƽ��״̬�ı�־��(����)
3C(g)+ D(g)���ݻ�������ܱ������н���,�ﵽ��ѧƽ��״̬�ı�־��(����) N2O4(��ɫ)�Ŀ��淴Ӧ��,����״̬һ������ƽ��״̬����(����)��
N2O4(��ɫ)�Ŀ��淴Ӧ��,����״̬һ������ƽ��״̬����(����)�� 2PbSO4+2H2O
2PbSO4+2H2O 2NH3
2NH3 HCl+HClO
HCl+HClO 2C(g)��2D(g)����H��Q,2 minĩ�ﵽƽ�⣬����0.8 mol D��
2C(g)��2D(g)����H��Q,2 minĩ�ﵽƽ�⣬����0.8 mol D�� mol����ʹƽ��ʱ�����ʵ����ʵ���Ũ����ԭƽ����ͬ����Ӧ�ü���B________mol��
mol����ʹƽ��ʱ�����ʵ����ʵ���Ũ����ԭƽ����ͬ����Ӧ�ü���B________mol��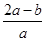 ��100%
��100% ��100%
��100% ��100%
��100% 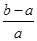 ��100%
��100% CO2(g)+H2(g),һ��ʱ���Ӧ�ﵽƽ�⡣�Ը�ƽ��״̬������ȷ����(����)
CO2(g)+H2(g),һ��ʱ���Ӧ�ﵽƽ�⡣�Ը�ƽ��״̬������ȷ����(����)