��Ŀ����
��2010?���գ���������ΪLiCoO2������ӵ���ѱ��㷺������Яʽ��Դ�����ܵ���Դ�ѷ����������һ����չ��
��1�����ʯ��LiFePO4��һ��DZ�ڵ�����ӵ���������ϣ�������ͨ����NH4��2Fe��SO4��2��H3PO4��LiOH��Һ������������Ӧ�����ó�����80����ո�����³��Ͷ��Ƶã�
�ٹ�������ӦͶ��ʱ��������NH4��2Fe��SO4��2��LiOH��Һֱ�ӻ�ϵ�ԭ����
�ڹ�������Ӧ�Ļ�ѧ����ʽΪ
�۸��³���ǰ������LiFePO4�м�����������̿�ڣ������ó��˿��Ը��Ƴ��ͺ��LiFePO4�ĵ��������⣬����
��2���Ͼ�����ӵ�ص�����������������Ҫ����LiCoO2������AI��Fe�ȣ���ͨ������ʵ�鷽�������ܡ�ﮣ�
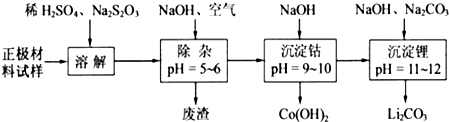
���������ܽ�����У�S2O32-��������SO42-��LiCoO2���ܽ�����з�Ӧ�Ļ�ѧ����ʽΪ
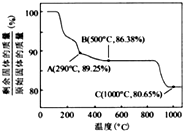
��Co��OH��2�ڿ����м���ʱ��������������¶ȵı仯����ͼ��ʾ����֪�ܵ��������������290��ʱ����ȫ��ˮ����1000��ʱ��ʣ�����ijɷ�Ϊ
��1�����ʯ��LiFePO4��һ��DZ�ڵ�����ӵ���������ϣ�������ͨ����NH4��2Fe��SO4��2��H3PO4��LiOH��Һ������������Ӧ�����ó�����80����ո�����³��Ͷ��Ƶã�
�ٹ�������ӦͶ��ʱ��������NH4��2Fe��SO4��2��LiOH��Һֱ�ӻ�ϵ�ԭ����
Fe2+�ڼ��������¸����ױ�����
Fe2+�ڼ��������¸����ױ�����
���ڹ�������Ӧ�Ļ�ѧ����ʽΪ
��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O
��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O
���۸��³���ǰ������LiFePO4�м�����������̿�ڣ������ó��˿��Ը��Ƴ��ͺ��LiFePO4�ĵ��������⣬����
�������O2��Ӧ����ֹLiFePO4�е�Fe2+������
�������O2��Ӧ����ֹLiFePO4�е�Fe2+������
����2���Ͼ�����ӵ�ص�����������������Ҫ����LiCoO2������AI��Fe�ȣ���ͨ������ʵ�鷽�������ܡ�ﮣ�


���������ܽ�����У�S2O32-��������SO42-��LiCoO2���ܽ�����з�Ӧ�Ļ�ѧ����ʽΪ
8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O
8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O
����Co��OH��2�ڿ����м���ʱ��������������¶ȵı仯����ͼ��ʾ����֪�ܵ��������������290��ʱ����ȫ��ˮ����1000��ʱ��ʣ�����ijɷ�Ϊ
CoO
CoO
�����ѧʽ������350��400�淶Χ�ڣ�ʣ�����ijɷ�ΪCo2O3��Co3O4
Co2O3��Co3O4
�����ѧʽ������������1���ٲ���ֱ�ӻ�ϵ�ԭ����Fe2+�ڼ��������¸����ױ�������
�ڸ����������Ϣ�������ķ�ӦΪ��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O��
�����Ŀ����е�O2������Fe2+����ֹFe2+��������
��2����ͨ�������Ϣ��֪LiCoO2��Na2S2O3������������ԭ��Ӧ����ӦΪ8LiCoO2+Na2S2O3+11H2SO4=4LiSO4+8CoSO4+Na2SO4+11H2O��
�ڸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬ͨ��������ݿ�����1000����Co��OH��2��ȫ�ֽ⣬�����CoO��
��350-400��ʱ�������������89.25%-86.38%֮�䣬����ͨ��������з�����
��290�棬n��Co����n��O��=
��[��89.25-100��
����16]=2��3���仯ѧʽΪCo2O3��
��500�棬n��Co����n��O��=
��[��86.38-100��
����16]=3��4���仯ѧʽΪCo3O4��
���Կ���ȷ����350-400��ʱ�Ļ�ѧʽΪCo2O3��Co3O4��
�ڸ����������Ϣ�������ķ�ӦΪ��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O��
�����Ŀ����е�O2������Fe2+����ֹFe2+��������
��2����ͨ�������Ϣ��֪LiCoO2��Na2S2O3������������ԭ��Ӧ����ӦΪ8LiCoO2+Na2S2O3+11H2SO4=4LiSO4+8CoSO4+Na2SO4+11H2O��
�ڸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬ͨ��������ݿ�����1000����Co��OH��2��ȫ�ֽ⣬�����CoO��
��350-400��ʱ�������������89.25%-86.38%֮�䣬����ͨ��������з�����
��290�棬n��Co����n��O��=
| 100 |
| 93 |
| 59 |
| 93 |
��500�棬n��Co����n��O��=
| 100 |
| 93 |
| 59 |
| 93 |
���Կ���ȷ����350-400��ʱ�Ļ�ѧʽΪCo2O3��Co3O4��
����⣺��1����NH4��2Fe��SO4��2��LiOH��Һ��Ӧ����Fe��OH��2��Fe��OH��2�ױ��������������Բ��ܽ�ֱ�ӻ�ϣ��ʴ�Ϊ��Fe2+�ڼ��������¸��ױ��������������𰸾��ɣ���
�ڸ��������Ϣ����NH4��2��H3PO4��LiOH��Һ������������Ӧ����LiFePO4��NH4HSO4��H2O����Ӧ�Ļ�ѧ����ʽΪ����NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O���ʴ�Ϊ����NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4��+2NH4HSO4+H2O��
�۸��³���ǰ������LiFePO4�м�����������̿�ڣ������ó��˿��Ը��Ƴ��ͺ��LiFePO4�ĵ��������⣬�������Ŀ����е�����������Fe2+����ֹFe2+���������ʴ�Ϊ���������O2��Ӧ����ֹLiFePO4�е�Fe2+��������
��2����������������Ҫ����LiCoO2������Al��Fe�ȣ�����ϡH2SO4��Na2S2O3��S2O32-��������SO42-�����л�ԭ�ԣ�����������ֻ��LiCoO2���������ԣ��뷴ӦNa2S2O3��Ӧ����CoSO4����Ӧ��ѧ����ʽΪ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O���ʴ�Ϊ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O��
�ڸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬����ԭʼ��������Ϊ100g����n��Co��=
mol��m��Co��=100��
g��
��1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=
��[��80.65-100��
����16]=1��1��ʣ�����ɷ�ΪCoO��
��350-400��ʱ�������������89.25%-86.38%֮�䣬����ͨ��������з�����
��290�棬n��Co����n��O��=
��[��89.25-100��
����16]=2��3���仯ѧʽΪCo2O3��
��500��n��Co����n��O��=
��[��86.38-100��
����16]=3��4���仯ѧʽΪCo3O4��
���Կ���ȷ����350-400��ʱ�Ļ�ѧʽΪCo2O3��Co3O4���ʴ�Ϊ��CoO��Co2O3��Co3O4��
�ڸ��������Ϣ����NH4��2��H3PO4��LiOH��Һ������������Ӧ����LiFePO4��NH4HSO4��H2O����Ӧ�Ļ�ѧ����ʽΪ����NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O���ʴ�Ϊ����NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4��+2NH4HSO4+H2O��
�۸��³���ǰ������LiFePO4�м�����������̿�ڣ������ó��˿��Ը��Ƴ��ͺ��LiFePO4�ĵ��������⣬�������Ŀ����е�����������Fe2+����ֹFe2+���������ʴ�Ϊ���������O2��Ӧ����ֹLiFePO4�е�Fe2+��������
��2����������������Ҫ����LiCoO2������Al��Fe�ȣ�����ϡH2SO4��Na2S2O3��S2O32-��������SO42-�����л�ԭ�ԣ�����������ֻ��LiCoO2���������ԣ��뷴ӦNa2S2O3��Ӧ����CoSO4����Ӧ��ѧ����ʽΪ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O���ʴ�Ϊ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O��
�ڸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬����ԭʼ��������Ϊ100g����n��Co��=
| 100 |
| 93 |
| 59 |
| 93 |
��1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=
| 100 |
| 93 |
| 59 |
| 93 |
��350-400��ʱ�������������89.25%-86.38%֮�䣬����ͨ��������з�����
��290�棬n��Co����n��O��=
| 100 |
| 93 |
| 59 |
| 93 |
��500��n��Co����n��O��=
| 100 |
| 93 |
| 59 |
| 93 |
���Կ���ȷ����350-400��ʱ�Ļ�ѧʽΪCo2O3��Co3O4���ʴ�Ϊ��CoO��Co2O3��Co3O4��
���������⿼���֪ʶ�Ƚ�ɢ���漰����Դ���ã��������ʡ��������̷�����ͼ��������������ȽϹ㣮

��ϰ��ϵ�д�
 53������ϵ�д�
53������ϵ�д�
�����Ŀ

 ��2010?���ն�ģ���������ٻ��š����ֵķз�����ķ���Prius����������϶������������õ綯������ȼ������߽���ƶ����֣��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬��
��2010?���ն�ģ���������ٻ��š����ֵķз�����ķ���Prius����������϶������������õ綯������ȼ������߽���ƶ����֣��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬��